MP Board Class 12th Chemistry Important Questions with Answers Guide Pdf Free Download रसायन विज्ञान in both Hindi Medium and English Medium are part of MP Board Class 12th Solutions. Here we have given NCERT Madhya Pradesh Syllabus MP Board Class 12 Chemistry Book Question Bank Solutions Rasayan Vigyan Pdf.
Students can also download MP Board 12th Model Papers to help you to revise the complete Syllabus and score more marks in your examinations.
MP Board Class 12th Chemistry Important Questions in English Medium
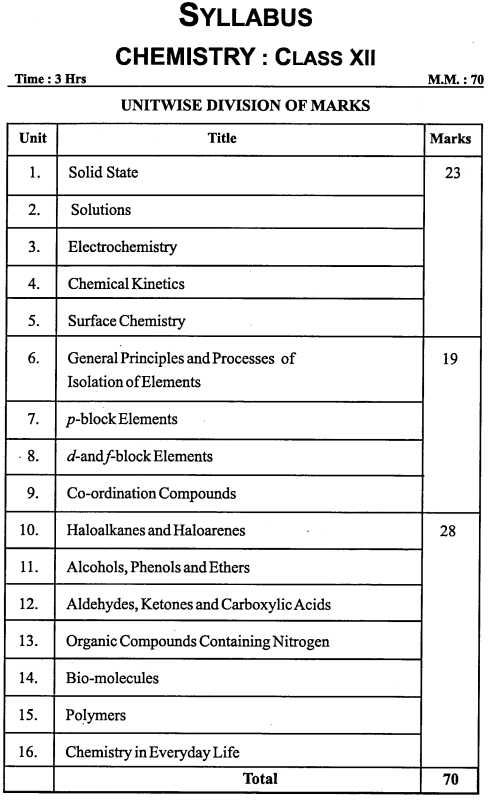
- Chapter 1 The Solid State Important Questions
- Chapter 2 Solutions Important Questions
- Chapter 3 Electrochemistry Important Questions
- Chapter 4 Chemical Kinetics Important Questions
- Chapter 5 Surface Chemistry Important Questions
- Chapter 6 General Principles and Processes of Isolation of Elements Important Questions
- Chapter 7 The p-Block Elements Important Questions
- Chapter 8 The d-and f-Block Elements Important Questions
- Chapter 9 Coordination Compounds Important Questions
- Chapter 10 Haloalkanes and Haloarenes Important Questions
- Chapter 11 Alcohols, Phenols and Ethers Important Questions
- Chapter 12 Aldehydes, Ketones and Carboxylic Acids Important Questions
- Chapter 13 Amines Important Questions
- Chapter 14 Biomolecules Important Questions
- Chapter 15 Polymers Important Questions
- Chapter 16 Chemistry in Everyday Life Important Questions
MP Board Class 12th Chemistry Important Questions in Hindi Medium
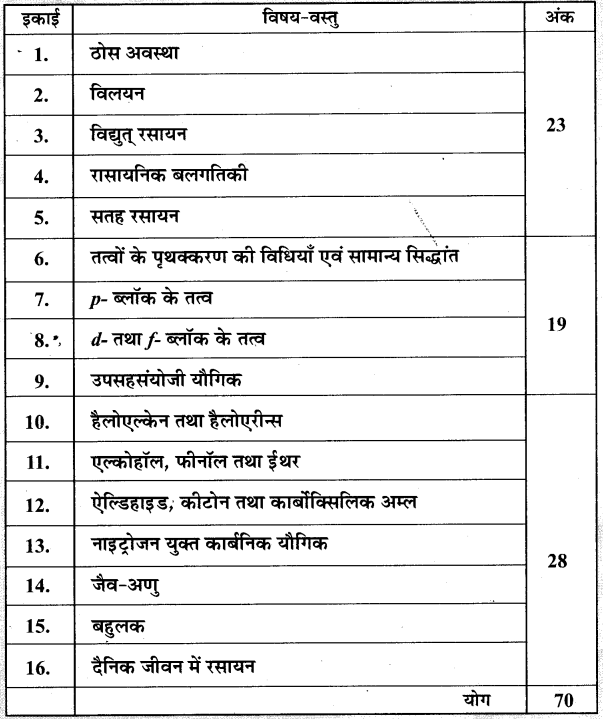
- Chapter 1 ठोस अवस्था Important Questions
- Chapter 2 विलयन Important Questions
- Chapter 3 वैद्युतरसायन Important Questions
- Chapter 4 रासायनिक बलगतिकी Important Questions
- Chapter 5 पृष्ठ रसायन Important Questions
- Chapter 6 तत्त्वों के निष्कर्षण के सिद्धान्त एवं प्रक्रम Important Questions
- Chapter 7 p-ब्लॉक के तत्त्व Important Questions
- Chapter 8 d एवं f-ब्लॉक के तत्त्व Important Questions
- Chapter 9 उपसहसंयोजन यौगिक Important Questions
- Chapter 10 हैलोऐल्केन तथा हैलोऐरीन Important Questions
- Chapter 11 ऐल्कोहॉल, फ़िनॉल एवं ईथर Important Questions
- Chapter 12 ऐल्डिहाइड, कीटोन एवं कार्बोक्सिलिक अम्ल Important Questions
- Chapter 13 ऐमीन Important Questions
- Chapter 14 जैव-अणु Important Questions
- Chapter 15 बहुलक Important Questions
- Chapter 16 दैनिक जीवन में रसायन Important Questions
MP Board Class 12th Chemistry Syllabus and Marking Scheme
Latest Syllabus and Marks Distribution Chemistry Class XII for the academic year 2019 – 2020 Year Examination.
Chemistry
Class XII
Time : 3 Hours.
Maximum Marks: 70
Unit Wise Division of Marks
1. Solid State
Classification of solids based on different binding forces : Molecular, ionic, covalent and metallic solids, amorphous and crystalline solids (elementary idea). Unit cell in two-dimensional and three-dimensional lattices, calculation of density of unit cell, packing in solids, packing efficiency, voids, number of atoms per unit cell in a cubic unit cell, point defects, electrical and magnetic properties. Band theory of metals, conductors, semiconductors and insulators and n and p type semiconductors.
2. Solutions
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, colligative properties : Relative lowering of vapour pressure, Raoult’s law, elevation in boiling point, depression in freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, van’t Hoff factor.
3. Electrochemistry
Redox reactions, conductance in electrolytic solutions, specific and molar con-ductivity, variations of conductivity with concentration, Kohlrausch’s law, elec-trolysis and law of electrolysis (elementary idea), dry cell- electrolytic cells and Galvanic cells; lead accumulator, EMF of a cell, standard electrode potential, Nemst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, fuel cells, corrosion.
4. Chemical Kinetics
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction : concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions); concept of collision theory (elementary idea, no mathematical treatment). Activation energy, Arrhenius equation. Surface Chemistry
5. Adsorption : Physisorption and chemisorption, factors affecting adsorption of gases on solids.
Catalysis: Homogeneous and heterogeneous, activity and selectivity; enzyme catalysis.
Colloidal state : Distinction between true solutions, colloids and suspension; lyophilic, lyophobic, multimolecular and macromolecular colloids; properties of colloids; Tyndall effect, Brownian movement, electrophoresis, coagulation. Emulsion : Types of emulsions.
6. General Principles and Processes of Isolation of Elements
Principle and methods of extraction; concentration, oxidation, reduction, elec-trolytic method and refining, occurrence and principles of extraction of aluminium, copper, zinc and iron.
7. p-block Elements
Group-15 elements : General indroduction, electronic configuration, occurrence, oxidation states, trends in physical and chemical properties; Nitrogen : Preparation, properties and uses; compounds of nitrogen, preparation and properties of ammonia and nitric acid, oxides of nitrogen (structure only); Phosphorus : Allotropic forms; compounds of phosphorus preparation and properties of phosphine, halides (PC13, PC15) and oxo-acids (elementary idea only).
Group-16 elements : General introduction, electronic configuration, oxidation states, occurrence, trends in physical and chemical properties; Dioxygen; preparation, properties and uses; classification of oxides; ozone, Sulphur: allotropic forms; compounds of sulphur; preparation, properties and uses of sulphur dioxide; sulphuric acid, industrial process of manufacture, properties and uses, oxo-acids of sulphur (structures only).
Group-17 elements : General introduction, electronic configuration, oxidation states, occurrence, trends in physical and chemical properties; compounds of halogens; preparation, properties and uses of chlorine, hydrochloric acid and interhalogen compounds, oxo-acids of halogens (structures only). Group-18 elements : General introduction, electronic configuration, occurrence, trends in physical ahd chemical properties, uses.
8. d- and f-block Elements
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first row transition metals : metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr207 and KMn04.
Lanthanoids : Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids : Electronic configuration, oxidation states and comparison with lanthanoids.
9. Co-ordination Compounds
Co-ordination compounds : Introduction, ligands, co-ordination number, colour, magnetic properties and shapes. IUPAC nomenclature of mononuclear co-ordination compounds. Bonding; Werner’s theory, VBT and CFT structure and stereo-isomerism, importance of co-ordination compounds (in qualitative inclusion, extraction of metals and biological system).
10. Haloalkanes and Haloarenes
Haloalkanes : Nomenclature, nature of C—X bond, physical and chemical properties, mechanism of substitution reactions, optical rotation.
Haloarenes : Nature of C—X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only). Uses and environmental effects of dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
11. Alcohols, Phenols and Ethers
Alcohols: Nomenclature, methods of preparation, physical and chemical prop-erties of (primary alcohols only); identification of primary, secondary and tertiary alcohols; mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols : Nomenclature; methods of preparation, physical and chemical prop-erties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers : Nomenclature; methods of preparation, physical and chemical properties, uses.
12. Aldehydes, Ketones and Carboxylic Acids
Aldehydes and Ketones : Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic ad-dition, reactivity of alpha hydrogen in aldehydes; uses.
Carboxylic acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
13. Organic Compounds Containing Nitrogen.
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines.
Cyanides and isocyanides : will be mentioned at relevant places in context. Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
14. Bio-molecules
Carbohydrates : Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration, oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); importance.
Proteins : Elementary idea of -amino acids, peptide bond, polypeptides, proteins, structure of proteins : primary, secondary, tertiary and quaternary structures (qualitative idea only), denaturation of proteins, enzymes. Hormones : elementary idea excluding structure.
Vitamins : Classification and functions.
Nucleic acids : DNA and RNA.
15. Polymers
Classification : Natural and synthetic, methods of polymerization (addition and condensation), copolymerization, some important polymers : Natural and synthetic like polythene, nylon, polyesters, bakelite, rubber. Biodegradable and non-biodegradable polymers.
16. Chemistry in Everyday Life
Chemicals in medjcines : Analgesics, tranquilizers, antiseptics, disinfectants, antimicrobials, antifertility drugs, antibiotics, antacids, antihistamines. Chemicals in food : Preservatives, artificial sweetening agents, elementaiy idea of antioxidants.
Cleansing agents : Soaps and detergents, cleansing action.
We hope the given MP Board Class 12th Chemistry Important Questions with Answers Guide Pdf Free Download रसायन विज्ञान in both Hindi Medium and English Medium will help you. If you have any queries regarding NCERT Madhya Pradesh Syllabus MP Board Class 12 Chemistry Book Question Bank Solutions Rasayan Vigyan Pdf, drop a comment below and we will get back to you at the earliest.
