MP Board Class 12th Chemistry Important Questions Chapter 8 The d-and f-Block Elements
The d-and f-Block Elements Important Questions
The d-and f-Block Elements Objective Type Questions
Question 1.
Choose the correct answer:
Question 1.
General electronic configuration of transition element is :
(a) (n – 1)d1 – 10 ns1
(b) (n – 1)d10ns2
(c) (n – 1)d1d1 – 10ns2
(d) (n – 1)d5ns1.
Answer:
(c) (n – 1)d1d1 – 10ns2
Question 2.
Reason of Lanthanide contraction is :
(a) Negligible screening effect of f – orbitals
(b) Increasing nuclear charge
(c) Decreasing nuclear charge
(d) Decreasing screening effect.
Answer:
(a) Negligible screening effect of f – orbitals
Question 3.
Chromyl chloride test confirms the presence of :
(a) Cl–
(b) SO42-
(c) Cr3+
(d) Cr3and Cl–.
Answer:
(a) Cl–
![]()
Question 4.
Formula of Mohr’s salt is :
(a) FeSO4.7H2O
(b) FeSO4.(NH4)2SO4.6H2O
(c) CU(OH)2.CUCO3.6H2O
(d) Fe2O3.3H2O.
Answer:
(b) FeSO4.(NH4)2SO4.6H2O
Question 5.
The outer electronic configuration of chromium is :
(a) 4s1, 3d5
(b) 4s2, 3d4
(c) 4s0, 3d6
(d) 4s2,3d5.
Answer:
(a) 4s1, 3d5
Question 6.
The equivalent weight of KMnO4 is alkaline medium will be :
(a) 31.60
(b) 52.66
(c) 79.00
(d) 158.00.
Answer:
(d) 158.00.
Question 7.
The Lanthanide which is widely used :
(a) Lanthanum
(b) Nobelium
(c) Thorium
(d) Cesium.
Answer:
(d) Cesium.
Question 8.
Electronic configuration of Gadolinium is :
(a) [Xe]4f6,5d9,6s2
(b) [Xe]4f7,5d16s2
(c) [Xe]4f3,5d5,6s2
(d) [Xe]4f6,5d2,6s2.
Answer:
(b) [Xe]4f7,5d16s2
Question 9.
In 3d series which element shows highest oxidation state :
(a) Mn
(b) Fe+2
(c) Ni
(d) Cr.
Answer:
(a) Mn
Question 10.
Fe, Co, Ni are magnetic substance of which type : (MP 2018)
(a) Paramagnetic
(b) Ferromagnetic
(c) Diamagnetic
(d) Antiferromagnetic.
Answer:
(b) Ferromagnetic
![]()
Question 11.
Number of unpaired electrons in Fe+2 ion is :
(a) 0
(b) 4
(c) 6
(d) 3.
Answer:
(b) 4
Question 12.
In which of the compounds Mn shows highest oxidation state :
(a) K2MnO4
(b) KMnO4
(c) MnO2
(d) Mn3O4.
Answer:
(b) KMnO4
Question 13.
The atomic radius and ionic radius of Zr and Hf are similar due to :
(a) Diagonal relationship
(b) Both are present in same group
(c) Lanthanide contraction
(d) Similar chemical properties.
Answer:
(c) Lanthanide contraction
Question 14.
Transition elements are coloured due to :
(a) Paired electron in d – orbital
(b) Paired electron in f – orbital
(c) Unpaired electron in d – orbital
(d) None of these
Answer:
(c) Unpaired electron in d – orbital
Question 15.
Stability of ferric ion is due to :
(a) Half filled d – orbital
(b) Half filled f – orbital
(c) Completely filled d – orbital
(d) Completely filled f – orbital.
Answer:
(a) Half filled d – orbital
Question 2.
Fill in the blanks :
- Metals Fe, Co, Ni are known as …………………….
- Ionic size of trivalent cations are ……………………. with increase in atomic numbers.
- The transition metals having lower oxidation state shows ……………………. nature.
- K2Cr2O7 is a strong ……………………. agent, which gives nascent oxygen.
- Zn shows only ……………………. oxidation state.
- f – block elements are known as ……………………. elements.
- Transition elements and their compounds act as …………………….
- General electronic configuration of inner transition element is …………………….
- Chemical form of Potassium manganate is …………………….
- d – block elements are also known as …………………….
Answer:
- Ferrous metals
- Decreases
- Basic
- Oxidising, 3
- +2
- Inner transition,
- Catalyst
- (n – 2)f1 – 14 (n – 1)d1 – 2(n – 1)d1 – 2ns2
- K2MnO4
- Transitional Elements.
Question 3.
State true or false :
- Mercury is liquid and its oxidation state is +1 and +2.
- Higher oxidation state of transition elements are acidic in nature.
- Lanthanides and Actinides both are transition elements.
- In all transition elements normal oxidation state is +2.
- Zn, Cd, Hg represent variable oxidation state.
- Cu+2 ion is colourless and diamagnetic.
- Plutonium used as fuel in nuclear reaction and in formation of atomic bomb.
- Transition elements easily form interstitial compounds.
Answer:
- True
- True
- False
- True
- False
- False
- True
- True.
Question 4.
Match the following :

Answer:
- (f)
- (g)
- (e)
- (c)
- (b)
- (d)
- (a)
![]()
5. Answer in one word / sentence :
- Which is colourless Cu2+or Cu+?
- In a reaction KMnO4 is replaced by K2MnO4 then what will be change in oxidation state of Mn?
- Which series shows higher oxidation state lanthanides or actinides?
- Which oxidation state of lanthanum is most stable?
- Write the equivalent weight of K2Cr2O7 in acidic medium.
- How many unpaired electrons are present in Fe3+?
- Give the name of oxidising agent used in chromyl chloride test.
- Out of d – block elements, Zn does not show variable valencies, why?
- Which is the most important oxidation state of Cu?
- d – block elements can be divided into how many series?
- What is Lunar caustic?
- In d – block elements Zn does not exhibit variable oxidation state. Why?
- What is the alkaline solution of HgCl2 and KI known as?
Answer:
- Cu+2
- 1
- Actinides
- +3
- 49
- 5
- K2Cr2O7
- Completely filled ‘d’ orbitals
- +2
- 2
- AgNO3 (Silver nitrate)
- Due to fully filled d – orbitals
- Nessler’s reagent.
The d-and f-Block Elements Very Short Answer Type Questions
Question 1.
Actinide contraction is greater from element to element than lanthanide contraction. Why? (NCERT)
Answer:
This is due to poor shielding effect by 5f electrons in the actinoids than that of 4f electrons in the lanthanoids.
Question 2.
Explain Cu+ is colourless while Cu+2 is coloured.
Answer:
If a transition metal contain unpaired electron, it shows paramagnetism and forms coloured compound. In Cu+d – orbital is partially filled (3d9) thus Cu+ is colourless and diamagnetic while Cu+2 is coloured and paramagnetic.
Question 3.
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 oxidation state? (NCERT)
Answer:
Mn+2 has stable electronic configuration [Ar]4r03d5 and they do not easily change to Mn+3, Fe+2 [Ar] 4s03d6 on oxidation forms Fe+3 [Ar] 4s03d5 a more stable configuration.
Question 4.
What are interstitial compounds? Why are such compounds well known for transition metals? (NCERT)
Answer:
Most of the transition elements form interstitial compounds at high temperature with atoms of non – metallic elements like H,B,C,N, Si etc. Small atoms of these non – metallic elements fit in the interstitial voids of crystal lattice of transition elements. These are called interstitial compounds.
![]()
Question 5.
What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses. (NCERT)
Answer:
An alloy is a homogeneous mixture of two or more metals or metals and non – metals. An important alloy contains lanthanoid metal is mischmetal which contains 50% Cerium and 25 % Lanthanum, with small amounts of Nd (Neodymium) and Pr (Praseodymium). It is used in Mg – based alloy to produce bullets, shell and lighter flints.
Question 6.
Ti2+, V2+ and Cr2+ are strong reducing agents. Why?
Answer:
For Ti2+, V2+ and Cr2+, values of M2+/M is negative which justify that they are strongly reducing.
Question 7.
Write the unit of magnetic moment.
Answer:
Bohr Magneton (BM).
The d-and f-Block Elements Short Answer Type Questions
Question 1.
Wnat is lanthanoid contraction? What are the consequents of lanthanoid contraction? (NCERT)
Answer:
Interesting feature of the atomic size of lanthanides is that on moving down the group steady decrease in atomic size is observed. The shape of f – orbital is in such a way that its shielding effect is minimum, there fore on addition of extra electron in f – subshell only attractive force increases. The steady decrease (contraction) in size of fourteen lanthanide elements (La3+1.06 Å to Lu3+ 0.8 Å) by a value of about 0.2Å is known as lanthanide contraction.
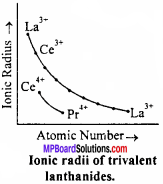
Reason:
1. The new electrons in lanthanides instead of going to outermost shell enters (n – 2)f – suborbital as a result of which force of attraction increases between electron and nucleus due to which atom or ion contracts.
2. Electron entering in (n – 2)f – suborbital have negligible or zero shielding effect over electrons present in the last orbit. In addition the shape of f – suborbital is not favourable for the shielding effect of electrons. Thus, lanthanide contraction occur.
Consequences of lanthanide contraction :
1. Change in the properties of lanthanides : Due to lanthanide contraction, little change occurs in the properties of lanthanides. So it is very difficult to obtain them in pure state.
2. Influence over the properties of other elements : Lanthanide contraction have an important influence over the element present before and after it e.g., there is difference in properties of Ti and Zr while Zr and Hf have similar properties.
Question 2.
What are Transition elements? They show metallic character. Why?
Answer:
Elements whose atoms in their ground state or ions in their common oxidation states have incomplete or partially filled d – orbitals are called transitional elements. They are in group 2 to 13. Example : Fe, Ni, Co, etc.
General formula : (n – 1)d1 – 10ns1 – 2
Metallic character of an element depends on its tendency to form cation by loosing one or more electrons from its atom. All transitional elements are metals because they contain one or two electrons in their outermost shell which can be easily lost due to low ionisation energy. Thus, they are metallic in nature.
![]()
Question 3.
Why do transition metals exhibit variable oxidation states?
Answer:
Transition metals exhibit variable valency because the energy subshell (n – 1 )d and ns are very close. Thus, possibility to lose electrons from ns subshell as well as from (n -1 )d subshell is very much if there are unpaired electrons. So oxidation states of these metals may increase. In these elements Mn shows maximum variable valencies.
Question 4.
Transition elements form alloy easily. Explain.
Answer:
It is the homogeneous mixture of two or more metals or metals with non – metals. Alloys are made to confer the property of metals. Transition elements have great tendency to form alloys because these elements have similar atomic size and can mutually substitute their positions in their crystal lattice. Alloys are comparatively hard and have higher m.p. than the elements from which they are made.
Question 5.
The radius of Fe2+ ion is smaller than the radius of Mn2+ion, why?
Answer:
The atomic number of Fe (26) is more than the atomic number of Mn (25). Due to higher value of atomic number, iron nucleus contains more protons. Hence the force of attraction between the nucleus and the electrons of outermost orbit is more. Due to strong attractive force of the nucleus the electron cloud is pulled inwards which results in smaller size of Fe2+ ion as compared to Mn2+ ion.
Question 6.
- Transition metals possess the ability to form complex compounds. Explain.
- Zn, Cd and Hg do not show the properties of Transition elements.
- Why is Ti known as a wonder metal?
Answer:
1. Cause of formation of complex compounds by Transition metals :
- Small size of ions of these elements and high nuclear charge due to which these ions attract ligands.
- They possess vacant d – orbitals in order to accomodate the electron pair donated by ligand.
2. Elements in which (n – 1) d – orbital is partially filled are known as Transition elements Whereas in Zn [3d104s2], in Cd [4d10 5s2] and in Hg [5d106s2] state is found. Therefore, these do not show the properties of Transition elements.
3. Titanium is a shining white metal. It is extended strong (harder than steel), has high m.p. Good conductor of electric current resistant to corrosion and light metal. Due to all these qualities, it is called wonder metal.
Question 7.
TiO2 is white whereas TiCl3 is violet, why?
In first transitional series paramagnetism increases till Cr then it starts de – creasing. Why?
Answer:
1. In TiO2, Ti is in +4 oxidation state (3d04s0) having a vacant rf-orbital hence there is no d – d transition and it is white. On the other hand, in TiCl3, Ti is in +3 oxidation state (3d14s0) having one unpaired electron in its 3d – orbital, hence it is coloured.
2. In first transitional series, the number of unpaired electrons till Cr (3d5) increases and then due to pairing the number of unpaired electrons decreases. Thus, due to this at first paramagnetismjacreases till Cr and then it decreases.
![]()
Question 8.
Write five differences between Lanthanide and Actinide.
Answer:
Differences between Lanthanides and Actinides Elements :
Lanthanides Elements:
- Lanthanides show oxidation state of + 3 mainly and +2 and +4 in few compounds.
- Tendency to form complex compound is low.
- Lanthanide compounds are less basic than actinide compounds.
- These do not form oxo – ions.
- Except promethium all are non – radioactive elements.
- Last electron enters in 4f – subshell.
Actinides Elements:
- Actinides show + 3 oxidation state together with + 4, + 5 and +6 in all compounds.
- Tendency to form complex compund is more than lanthanides.
- Actinide compounds are more basic.
- Actinides form oxo – ions as UO2+, NpO+, PuO2+, etc.
- All actinides are radioactive.
- Last electron enters in 5f – subshell.
Question 9.
Write chromyl chloride test with equation.
Answer:
Chromyl Chloride Test:
1. When a metal chloride is heated with solid potassium dichromate and cone. H2SO4 orange coloured vapours of chromyl chloride are formed.
K2Cr2O7 + 6H2SO4 + 4KCl → 2CrO2Cl2 ↑ + 6KHSO4 + 3H2O
2. When these fumes are passed in sodium hydroxide solution, yellow solution of sodium chromate is obtained. When lead acetate is added to it in presence of acetic acid yellow precipitate of lead chromate is obtained.
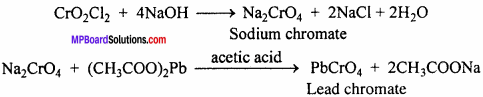
Question 10.
Write any five main differences between d – and f – Block elements.
Answer:
Differences between d and f – Block Elements :
d – Block Elements:
- Two shells n and (n – 1) are incomplete.
- Last electron enters the d – orbital of penultimate shell.
- d – block elements are normally called Transitional element.
- d – block elements are available in nature.
- These elements exhibit variable oxidation state.
- These elements are stable.
f – Block Elements:
- Three shells n, (n – 1) and (n – 2) are incomplete.
- Last electron enters the orbital of antipenultimate (n – 2) shell.
- f – block elements are normally called Inner Transitional element.
- f – block elements are very rare. Therefore, they are known as Rare Earth elements.
- These elements also exhibit variable oxidation state.
- These elements are less stable and many are radioactive.
![]()
Question 11.
Explain giving reasons: (NCERT)
- Transition metals and many of their compounds show paramagnetic behaviour.
- The enthalpies of atomisation of the transition metals are high.
- The transition metals generally form coloured compounds.
- Transition metals and their many compounds act as good catalyst.
Answer:
1. Paramagnetic substance is one which is attracted by magnetic field. It arises due to presence of unpaired electron in atom, ion or molecule. Most of the transition elements and compounds are paramagnetic in nature. This is due to fact that transition elements involve partially filled d – subshell and their atom and ion contain unpaired electron.
2. Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high.
3. The colour of transitional metal ions is due to partially filled (n – 1 )d orbitals. In transitional metal ions which contain unpaired d electrons, transition of electrons takes place from one d – orbital to another d – orbital. During this transition it absorbs some radiation of visible light and reflects the remaining radiation in the form of coloured light. Thus, the colour of the ion is complementary to the colour absorbed by it.
For example:
[Cu(H2O)6]2+ ion appears blue because it absorbs the red colour of the visible light for electron promotion and reflects its complementary blue colour.
Colour of some ions:
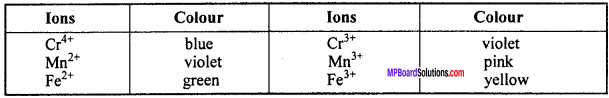
4. Transition elements act as good catalysts in chemical reaction in the hydrogenation of Ni metal, in contact process of manufacture of SO3, Pt and in manufacture of NH3 by Haber process Fe acts as catalyst. In the method of preparation of O2 by heating KClO3, MnO2 acts as catalyst.
Question 12.
How would you account for the following : (NCERT)
- Of the d4 species, Cr2+ is strongly reducing while manganese(III) is strongly oxidising.
- Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.
- The d1 configuration is very unstable in ions.
Answer:
1. Cr+2 is reducing in nature as its configuration changes from d4 to d3 (A stable configuration having half filled t2g orbitals). On the other hand, Mn+3 is oxidising in nature as the configuration changes from d4 to d5 (A stable configuration having half filled t2gto e orbitals)
2. Strong ligands force Cobalt (II) to lose One more electron from 3d – subshell and thereby induce d2sp3 – hybridisation.
3. The ions with dl configuration try to lose the only electron on d – subshell in order to acquire stable inert gas configuration.
Question 13.
Compare the chemistry of actinoids with that of the lanthanoids with special reference to: (NCERT)
- Electronic configuration
- Atomic and ionic sizes
- Oxidation state and
- Chemical roactivity.
Answer:
Differences between Lanthanoids and Actinoids :
Lanthanoids:
- Differentiating or last electrons enter in 4f – sub – shell of (n – 2) orbit.
- These elements come after lanthanum so these are called lanthanoids.
- Common oxidation state is +3, other oxidation states are +2 and +4 also.
- Atomic or ionic radius decreases gradually and this is called lanthanide contraction.
- Lanthanoids have smaller tendency to form complexes.
- Lanthanoids do not form oxo – ions.
- Compounds of lanthanoids exhibit less basic in nature.
- Lanthanoids are not radioactive except Promethium.
- Except Pm, other lanthanoids are present in nature in abundance comparatively more than iodine.
Actinoids:
- Differentiating or last electrons enter in 5f – sub – shell of (n – 2) orbit.
- These elements come after actinium so these are called actinoids.
- Common oxidation state in actinoids is also +3 but other oxidation states are higher, example +4, +5, +6 and +7.
- Atomic or ionic radius also decreases gradually and steadily and this is qallejj actinoid contraction.
- Actinoids have comparatively higher tendency of complex formation.
- Oxo – ions are formed. example UO2+,PuO2+, UO+, etc.
- Compounds of actinoids are more basic in nature.
- All the actinoids are radioactive.
- Most of these are not found in nature and are artificially prepared.
Question 14.
What are Inner Transition elements? (NCERT)
Answer:
These are the elements which contain (n-2)f and (n-1)d incomplete orbitals or in which electron enter in the antipenultimate (two energy levels below the outermost orbital) orbital. These are so called because these are found within the transition elements. There are two types of inner transition elements :
(i) Lanthanides series :
The 14 elements after Lanthanum (La57)
i.e., 58Ce – 71Lu are called lanthanides.
(ii) Actinide series : 14 elements after Actinide (AC89) i.e., Th90 to LW103.
The d-and f-Block Elements Long Answer Type Questions
Question 1.
Describe the preparation of K2Cr2O7 from chromite ore and explain the reactions of K2Cr2O7 with acidic FeSO4, KI and H2S.
Answer:
(A) Preparation:
It is prepared from chromite ore or ferrochrome of chrome iron FeCr2O4 (FeO.Cr2O3). Different steps involved in the process are as follows :
1. Preparation of sodium chromate:
The ore is finely powdered, mixed with sodium carbonate and quick lime and then roasted (heated to redness) in a reverberatory furnace in presence of excess of air when sodium chromate (yellow in colour) is formed with the evolution of CO2. Quick lime is added to keep the mass porous and thus facilitates oxidation.
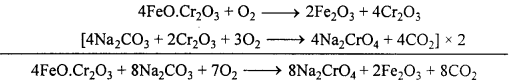
The roasted mass is the extracted with water when sodium chromate dissolves com-pletely leaving behind ferric oxide.
2. Conversion of sodium chromate to sodium dichromate:
Sodium chromate is extracted with water and acidified with sulphuric acid to get sodium dichromate.
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
On concentration the less soluble sodium sulphate Na2SO4.10H2O crystallizes out. This is filtered hot and allowed to cool when sodium dichromate Na2Cr2O7.2H2O separates on standing.
3. Conversion of sodium dichromatic into potassium dichromate:
Hot concentrated solution of sodium dichromate is treated with requisite amount of potassium chloride when potassium dichromate being less soluble crystallizes out on cooling.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
(B) Reaction of K2Cr2O7 with acidic FeSO4, KI and H2S :
(i) It oxidizes ferrous sulphate to ferric sulphate.
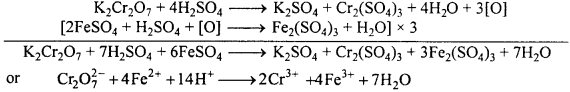
(ii) It liberates I2 from KI.
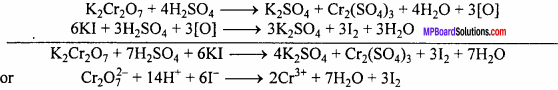
These reactions are used in the estimation of iodine and ferrous ion in volumetric an-alysis.
(iii) It oxidizes SO2 to sulphuric acid.
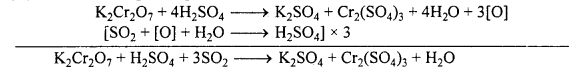
(iv) It oxidizes H2S to sulphur.
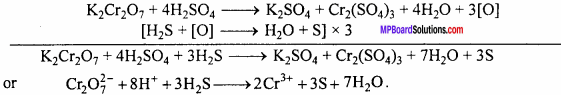
Question 2.
Explain the oxidizing property of KMnO4 in acidic, neutral and alkaline medium giving two examples each.
Answer:
KMnO4 acts as strong oxidizing agent in acidic, neutral and alkaline medium. In acidic medium : It oxidizes in presence of dilute H2SO4 and get reduced.
2KMnO4 +3H2SO4 → K2SO4 +2MnSO4 +3H2O + 5[O]
Example:
1. It oxidizes ferrous salt into ferric salt.
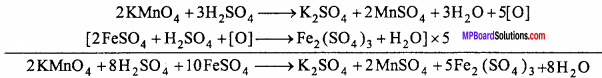
2. It oxidizes oxalate to CO2 :
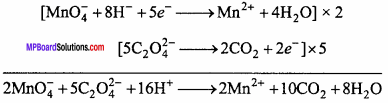
2KMnO4 + 3H2SO4 + 5C2H2O4 → K2SO4 + 2MnSO4 + 8H2O + 10CO2
3. It oxidizes iodide ion to iodine :
2KMnO4+10KI+8H2SO4 → 6K2SO4 + 2MnSO4+ 8H2O + 5I2
4. It oxidizes nitrites to nitrates :
2KMnO4 + 3H2SO4 + 5NaNO2 → 2MnSO4 + K2SO4 + 5NaNO3 + 3H2O
In neutral medium:
In this medium, the reaction begins with neutral ethylene glycol but this does not give neutral reaction because KOH formed in the reaction makes basic in nature.
2KMnO4 + H2O → 2KOH + 2MnO2 + 3[O]
Example:
1. It oxidizes manganous sulphate to manganese dioxide.
2KMnO4 + 3MnSO4 + 2H2O → 5MnO2 + K2SO4 + 2H2SO4
2. It oxidizes hydrogen sulphide to sulphur.
2KMnO4 + 4H2S → 2MnS + K2SO4 + 4H2O + S
In alkaline medium:
In alkaline medium, reduces to MnO2 and gives 3 nascent oxygen.
Example:
1. It oxidizes ethylene to ethylene glycol

2. It oxidizes iodide to iodate.
![]()
KMnO4 gives more number of nascent oxygen in acidic medium than in alkaline medium due to which it acts as stronger oxidizing agent in acidic medium.
![]()
Question 3.
Describe the preparation of KMnO4 from pyrolusite and explain its oxidising properties in acidic, basic and neutral medium by suitable example. (MP 2009 Set B, 17)
Answer:
Preparation:
Potassium permanganate is prepared from manganese dioxide. On a large scale, it is prepared from the mineral pyrolusite. The process involves the following steps:
1. Conversion of MnO2 into potassium manganate:
The finely powdered pyrolusite mineral is fused with potassium carbonate or potassium hydroxide in presence of atmospheric oxygen or an oxidising agent such as potassium nitrate or potassium chlorate. The fused mass turns green due to the formation of potassium manganate.
The fused mass turns green due to the formation of potassium manganate.
2MnO2 + 2K2CO3 + O2 → 2K2MnO4 + 2CO2
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
MnO2 + 2KOH + KNO3 → K2MnO4 + KNO2 + H2O
3MnO2 + 6KOH + KClO3 → 3K2MnO4 + KCl + 3H2O
2. Oxidation of potassium manganate into potassium permanganate :
(i) Chemical oxidation:
The fused mass is extracted with water and the solution is filtered. The green solution is then converted to potassium permanganate by bubbling carbon dioxide, chlorine or oxygen through it.
3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 ↓ + 2K2CO3
2K2MnO4+ Cl2 → 2KMnO4 + 2KCl
2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2
The purple solution of potassium permanganate thus obtained is concentrated when it deposits dark purple, needle like crystals having a metallic lustre.
(ii) Electrolytic oxidation: Nowadays, it is largely manufactured by the electrolytic oxidation of the manganate. The manganate solution is electrolysed between iron electrodes separated by diaphragm. The oxygen evolved at the anode converts manganate to permanganate.
2K2MnO4 + H2O + [O] → 2KMnO4 + 2KOH
MnO42- + e– Oxidation (At anode)
2K+ + 2e–→ 2K Reduction (At cathode)
2K + 2H2O → 2KOH + H2
After the oxidation is completed, the solution is filtered and evaporated under controlled condition to obtain the crystals of potassium permanganate.
(i) Acidified KMnO4 solution oxidizes Fe(II) ions to Fe(III) ions i.e. ferrous ions to ferric ions.
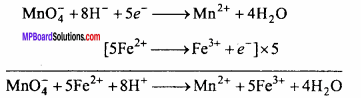
(ii) Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
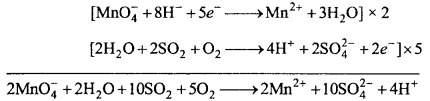
(iii) Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.