MP Board Class 12th Chemistry Solutions Chapter 5 Surface Chemistry
Surface Chemistry NCERT Intext Exercises
Question 1.
Why are substance like platinum and palladium used for carrying electrolysis of aqueous solutions ?
Answer:
These metals are not attacked by the ions of the electrolytes or the products of electrolysis. Electrodes of such metals are called inert electrodes.
Question 2.
Why does physisorption decreases with the increase of temperature ?
Answer:
It is an exothermic process
![]()
Thus, when temperature is raised, reaction proceeds in backward direction and gas adsorbed gets released. It is accordance to Le-Chatelier’s principle.
Question 3.
Why are powdered substances more effective adsorbent than their crystal line forms ?
Answer:
Powdered form has greater surface area. Greater the surface area, greater is the adsorption.
Question 4.
Why is it necessary to remove CO when ammonia is obtained by Haber’s process ?
Answer:
For the preparation of ammonia in Haber’s process solid catalyst is used. It is necessary to remove the CO produced because the gas reacts with iron and forms Fe(CO)5 which exist in liquid state at the temperature of the chamber and obstructs in the production of NH3 because at high temperature CO and H2 react by which production of ammonia decreases.
In short CO acts as poison for the iron catalyst used in the process. It is therefore, necessary to remove CO.
Question 5.
Why is the ester hydrolysis slow in the beginning and becomes faster after sometime ?
Answer:
CH3COOC2H5 + H2O → CH3COOH + C2H5OH
Infact, this reaction is catalysed by an acid (H+). Acetic acid formed during the reac¬tion catalyses the hydrolysis of ester. Therefore, reaction becomes fast. It is called autocatalyst.
Question 6.
What is the role of desorption in the process of catalysis ?
Answer:
The product formed leaves the surface of catalyst and makes it available for adsorption of mole reactants molecules.
Question 7.
What modifications can you suggest in the Hardy-Schulze law ?
Answer:
The coagulating ion has charge opposite to that on the colloidal particles. Thus, when coagulating ion is added to colloidal solution, the change on colloidal particle is neutralised and coagulation occurs. According to Hardy-Schulze rule, greater the volume of the oppositely charged ions of the electrolyte faster is the coagulation the Hardy-Schulze law, consider the coagulation of colloidal sols because of neutralisation of charge on colloidal particles. We know that neutralisation and coagulation also takes place by mixing two oppositely charged colloidal solutions.
Thus, Hardy-Schulze law should also include the following when oppositely charged sols are mixed in right proportions, they neutralize charges of each other and undergo coagulation.
Question 8.
Why is it essential to wash the precipitate with water before estimating it quantitatively ?
Answer:
Some amount of impurities remains adsorbed on the surface of the precipitate. It is essential to remove the sticking impurities by washing with water.
![]()
Surface Chemistry NCERT TextBook Exercises
Question 1.
Distinguish between the meaning of the terms adsorption and absorption.
Answer:
Differences between Adsorption and Absorption :
Adsorption:
- It is a process as a result of which one substance get concentrated only on the surface of the other.
- Concentration of adsorbate on the surface of adsorbent is different than in the bulk.
- It is a surface phenomenon.
Example : Adsorption of water vapour on silica gel.
Absorption:
- It is a process as a result of which one substance gets uniformly distributed in the volume of the other.
- Concentration is uniform in the entire solid system.
- It is a bulk phenomenon.
Example : Adsorption of water vapour by dry calcium chloride.
Question 2.
What is the difference between physisorption and chemisorption ?
Answer:
Differences between Physical adsorption and Chemical adsorption :
Physical adsorption:
- Low value of enthalpy of adsorption (20-40 kJ mol-1)
- This type of adsorption involves weak vanderWaals’ forces between adsorbent and adsorbate.
- Usually takes place at low temperature and decreases with increase in temperature.
- It is reversible in nature.
- The extent of adsorption is approximately related to the case of liquification of the gas.
- Forms multimolecular layers.
- On increasing pressure, adsorption also increases.
Chemical adsorption:
- High value of enthalpy of adsorption (40-400kJmol-1 ).
- This type of adsorption involves strong forces of attraction due to chemical bond formation.
- Takes place at high temperature.
- It is irreversible.
- No such correlation.
- Forms monomolecular layers.
- No any effect of pressure.
Question 3.
Give reason why a finely divided substance is more effective as an adsorbent ?
Answer:
With the increase in surface area of adsorbent adsorption increases. Thus, in powdered state (finely divided substance) or in porous state surface area of metals is more. Therefore, adsorption is more in these states.
Question 4.
What are the factors which influence the adsorption of a gas on a solid ?
Answer:
Factors which affect the adsorption of gas on a solid are as follows :
- Nature of gas
- Surface area of adsorbent
- Pressure
- Temperature
- Activity of adsorbent.
Question 5.
What is an adsorption isotherm ? Describe Freundlich adsorption isotherm.
Answer:
The graph showing the extent of adsorption versus pressure at constant tempera¬ture is known as adsorption isotherm. Adsorption isotherm graphs are of two types :
(a) Freundlich isotherm graph.
(b) Langmuir isotherm graph.
Freundlich isotherm: Freundlich gave an experimental relation between the amount of gas adsorbed by unit mass of adsorbent with pressure at a definite temperature. This relation can be represented by the following equation :
\(\frac{x}{m}=\mathrm{K.P}^{1 / n}(n>1)\) …..(1)
Where m is the mass of gas adsorbed on adsorbent x at pressure P, K and n are con¬stants which depend on the nature of adsorbent and gas at a definite temperature.
These graphs indicate at the definite pressure, physical adsorption decreases with the increase in temperature.
Taking log of equation (1)
log \(\frac { x }{ m } \) = log K + \(\frac { 1 }{ n } \) log P …..(2)
When a graph is drawn by taking log \(\frac { x }{ m } \) on y-axis and log P on x-axis, a straight line is obtained. This verifies Freundlich isotherm.
Slope = \(\frac { 1 }{ n } \) and intercept on y-axis = log K
value of \(\frac { 1 }{ n } \) can be between 0 and 1, thus eqn. (2)
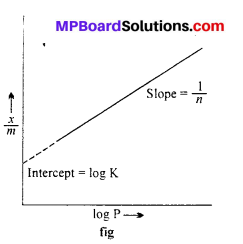
can be applicable only upto a limited extention of pressure.
(i) When \(\frac { 1 }{ n } \) = 0, \(\frac { x }{ m } \) = constant, then adsorption is independent of pressure.
(ii) When \(\frac { 1 }{ n } \) = 1, \(\frac { x }{ m } \) = Kp, \(\frac { x }{ m } \) α P, then change in adsorption is proportional to pressure.
Question 6.
What do you understand by activation of adsorbent ? How is it achieved ?
Answer:
It means increasing the adsorption power of an adsorbent; it is done (i) by increasing the surface area, (ii) by making the surface rough, (iii) by removing the gas already adsorbed.
Question 7.
What role does adsorption play in heterogeneous catalysis ?
Answer:
In heterogeneous catalysis, the reactants are generally gases while catalysts are solids. The reactant molecules are adsorbed on the surface of solid catalyst by physical adsorption or chemical adsorption. As a result, the concentration of the reactant molecules on the surface of the catalyst increases and hence the rate of reaction also increases.
Question 8.
Why is adsorption always exothermic ?
Answer:
Adsorption leads to decrease in disorder, thus ∆S = +ve. For the process to be performed value of ∆G should be -ve. Thus, in equation ∆G = ∆H – T∆S, ∆G will be negative when ∆H will be negative i.e., exothermic. Thus, adsorption is an exothermic process.
Question 9.
How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium ?
Answer:
Classification on the basis of physical state of dispersed phase and dispersion medium : Depending upon the physical state of dispersed phase and dispersion medium whether these are solids, liquids or gases, eight types of colloidal systems are possible. The examples of the various types of colloids and their typical names are given in Table.
It may be noted that a gas mixed with another gas forms a homogeneous mixture and therefore it is not a colloidal system.
Different Types of Colloidal Systems
| S.No. | Dispersed phase | Dispersion medium | System name | Example |
| 1. | Solid | Solid | Solid sol | Some coloured glasses, gemstone. |
| 2. | Solid | Liquid | Sol | Gold or silver sol, muddy water, paint, cell fluids. |
| 3. | Solid | Gas | Aerosol of solid | Smoke, dust. |
| 4. | Liquid | Solid | Gel | Jelly, curd, cheese |
| 5. | Liquid | Liquid | Emulsion | Milk, hair cream |
| 6. | Liquid | Gas | Aerosol of liquid | Fog, cloud, mist, insecticide spray. |
| 7. | Gas | Solid | Solid foam | Pumice-stone, foam, rubber cork |
| 8. | Gas | Liquid | Foam or froth | Froth, whipped cream, soap lather. |
Out of the different types of colloids, the most common are sols (solid in liquids), gels (liquid in solids) and emulsions (liquid in liquid).
Question 10.
Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Answer:
Effect of pressure: Adsorption is a reversible process and is accompanied by a decrease in pressure. Therefore, adsorption increases with an increase in pressure.
Effect of temperature: Adsorption is an exothermic process. Thus, in accordance with Le-Chatelier’s principle the mangnitude of adsorption decreases with an increase in temperature.
Question 11.
What are lyophilic and lyophobic sols ? Give one example of each type. Why are hydrophobic sols easily coagulated ?
Answer:
Lyophilic sols : Those sols in which there are forces of attraction between dispersed phase and dispersion medium, are called lyophilic sols. Lyophilic means liquid loving. They are directly prepared by mixing substances like gum, gelatine, starch with suitable dispersion medium like water, e.g., starch in water, albumin in water. They are reversible sols.
Lyophobic sols : The word ‘lyophobic’ means liquid hating. Substances like metals, their sulphides etc. when mixed with dispersion medium, do not form sols. They are prepared by special methods, e.g., Gold sol, As2S3 sol etc. In these sols, the particles of disperesed r phase have no affinity for dispersion medium and they are irreversible sols.
Reasons for coagulation of lyophobic sols : Lyophobic sols are easily precipited or coagulated on addition of small amounts of electrolytes, by heating or by shaking because they are not stable due to less force of attraction between dispersed phase and dispersion medium.
Question 12.
What is the difference between multimolecular and macromolecular col-loids ? Give one example of each. How are associated colloids different from these two types of colloids ?
Answer:
(i) Multimolecular colloids: In this type of colloids the colloidal particles consist of aggregates of atoms or small molecules with diameter of less than 1 nm. For example, a gold sol may contain particles of different sizes having several atoms of gold. Similarly sulphur sol contains about one thousand of S8 molecules. These are held together by van der Waals’ forces.
(ii) Macromolecular colloids : In macromolecular colloids the dispersed particles themselves are big molecules, usually polymers. The molecular masses of these macro molecules range from thousands to millions. Synthetic compounds e.g. polyethylene, polystrene, nylon, rubber etc. are macromolecules. Since these molecules have dimensions comparable to those of colloidal particles, there dispersions are known as macromolecular colloids. Most lyophillic sols are of this category.
(iii) Associated colloids (Micelles): There are certain substances which behave as normal, strong electrolytes at low concentration, but exhibit colloidal properties at higher concentrations due to the formation of aggregated particles. These are called Micelles. These substances are also called Associated Colloids. Surface active agepts like soaps and detergents are of this class.
Question 13.
What are enzymes ? Write in brief the mechanism of anzyme catalysis.
Answer:
Enzymes are complex nitrogeneous organic compounds present in living beings. Enzymes catalyse many biochemical reactions taking place inside the human body like digestion, i.e., why enzymes are also called biocatalysts. For example,
Inversion of cane sugar is catalysed by invertase.

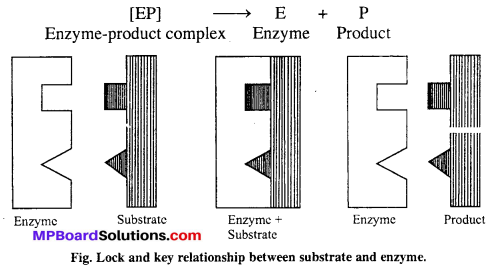
Mechanism of enzyme catalysis : There are present a number of cavities of characteristic shape on the surface of enzymes. The molecules of the reactant (substrate) which have complementary shape, fit into these cavities just as a key fits into a lock. An activated enzyme-substrate complex is formed which then decomposes to yield products. It involves following steps :
Step-I → Binding enzyme to substrate (reactant) to form a complex.
![]()
Step-II → Product formation from the complex
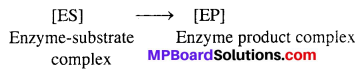
Step-III → Release of the product from the enzyme product complex.
Question 14.
How are colloids classified on the basis of:
(i) Physical states of components
(ii) Nature of dispersion medium and
(iii) Interaction between dispersed phase and dispersion medium ?
Solution:
Colloids can be classified on various bases as :
(i) On the basis of the physical state of the components (by components we mean the dispersed phase and dispersion medium). Depending on whether the components are solids, liquids, or gases, we can have eight types of colloids.
(ii) On the basis of the dispersion medium, sols can be divided as :
| No. | Dispersion medium | Name of sol |
| 1. | Water | Aquasol or hydrosol |
| 2. | Alcohol | AIcosol |
| 3. | Benzene | Benzeosol |
| 4. | Gases | Aerosol |
(iii) On the basis of the nature of the interaction between the dispersed phase and dispersion medium, the colloids can be classified as lyophilic (solvent attracting) and lyophobic (solvent repelling).
Question 15.
Explain, what is observed :
(i) When a beam of light is passed through a colloidal sol ?
(ii) An electrolyte, NaCl is added to hydrated ferric oxide sol ?
(iii) Electric current is passed through a colloidal sol ?
Answer:
(i) Scattering of light by colloidal particles takes place and path of the light becomes visible (Tyndall effect).
(ii) The positively charged colloidal particles of Fe(OH)3 get coagulated by the oppositely charged Cl– ions provided by NaCl.
(iii) On passing.electric current, the colloidal particles move towards the oppositely charged electrode where, they lose their charge and get coagulated. This is electrophoresis process.
Question 16.
What are emulsions ? What are their different types ? Give examples of each type.
Answer:
The colloidal system in which dispersed phase and dispersion medium both are liquid, is called emulsion.
Types of emulsions:
1. Oil in Water type (O/W) : In this type of emulsion small droplets of oil are dispersed in the water or dispersion medium, e.g., Milk, vanishing cream.

2. Water in Oil (W/O): In this type of emulsion, small quantity of water is dispersed in the form of droplets in oil (dispersion medium), e.g., Butter, Cod-liver.
Question 17.
What is demulsification ? Name two demulsifiers.
Answer:
The process of separation of constituent liquids of an emulsion is called demulsification. Demulsification can be done by centrifuging or boiling.
Question 18.
Action of soap is due to Emulsification or formation of micelle formation. Comment
Answer:
Cleaning action of soaps: Cleaning action of soap and detergents are based on miceller activity. Soaps are sodium salt of higher fatty acids e.g., sodium stearate which ionises as:
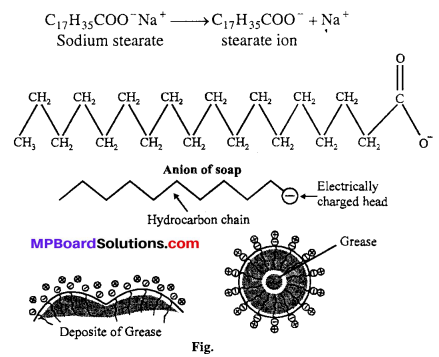
The anionic head of stearate ion (-COO–) is hydrophilic and hence has great affinity for water. The hydrocarbon part is hydrophobic and has great affinity for oil, grease etc.
When soap dissolves in water the anions (C17H35COO–) form micelle encapsulating oil or grease inside. These micelles are removed by rinsing with water. Thus the main function of soap is to convert oily and greasy dirt to colloidal particles by forming an emulsion. Soaps therefore, act as emulsifying agents. This mechanism of cleaning is also applicable to synthetic detergents like sodium lauryl sulphate CH3(CH2)11 SO–4 Na+. Repulsion of similar charge -COO– or -SO–4 covering each oil (grease) micelle prevents them to come together. Thus oil or grease remains in the suspension.
Question 19.
Give four examples of heterogeneous catalysis.
Answer:
Four examples of heterogeneous catalysis: (i) Oxidation of sulphur dioxide to form sulphur trioxide. In this reaction, Pt acts as catalyst.
![]()
(ii) Formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron.
![]()
This process is called the Haber’s process.
(iii) Ostwald’s process : Oxidation of ammonia to nitric oxide in the presence of platinum.
![]()
(iv) Hydrogenation of vegetable is in the presence of Ni.
![]()
Question 20.
What do you mean by activity and selectivity of catalysts ?
Answer:
(a) Activity of a catalyst: The activity of a catalyst is its ability to increase the rate of a particular reaction. Chemisorptions is the main factor in deciding the activity of a catalyst. The adsorption of reactants on the catalyst surface should be neither too strong nor too weak. It should just be strong enough to make the catalyst active.
(b) Selectivity of the catalyst: The abilitiy of the catalyst to direct a reaction to yield a particular product is referred to as the selectivity of the catalyst. For example, by using different catalyst, we can get different products for the reaction between H2 and CO.
Question 21.
Describe some features of catalysis by zeolites.
Answer:
- Zeolites are hydrated alumino-silicates. They have three-dimensional network structure. They contain water molecules in their pores,
- Zeolites are heated to remove water of hydration. The pores become vacant and zeolites are ready to act as catalyst.
- The size of the pores varies from 260 pm to 760 pm. This shows that only those molecules can be adsorbed in these pores whose size is small enough to enter these pores. Thus, zeolites as molecular sieve and the shape selective catalysts.
Question 22.
What is shape selective catalysis ?
Answer:
A catalyst reaction is very specific, which depends upon the pore structure of the catalyst and on the size of the reactant and the product. Molecules is called shape-selective catalysis. For example: catalyst by zeolite is shape-selective catalysis.The pore size present in the zeolite ranges from 260-740 pm. Molecules having a pore size more than this cannot enter the zeolite and undergo the reaction.
Question 23.
Explain the following terms :
(i) Electrophoresis
(ii) Coagulation
(iii) Dialysis
(iv) Tyndall effect.
Answer:
(i) Electrophoresis : Colloidal particles are either positively charged or negatively charged. When this colloidal solution is put in electric field, colloidal particles move towards the electrodes of opposite charge and on reaching there deposited and get precipitated As, AS2S3 particles being negatively charged move towards anode and precipitated there.
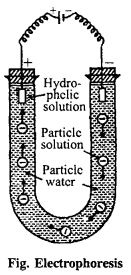
The movement of colloidal particles towards the electrodes of opposite charge is called electrophoresis.This electrophoresis is also known as cataphoresis and anaphoresis depending upon movement towards cathode and anode respectively.
(ii) Coagulation : Colloidal particles are either positively charged or negatively charged. It is seen that on adding some oppositely charged ions or electrolyte, which cancels the charge of colloidal particles and these particles aggregate and change into precipitates. Precipitation of colloidal particles by electrolytes is called coagulation.
(iii) Dialysis: The process of separating impurity of crystalloids from colloidal sol by means of diffusion of the former through an animal or vegetable membrane, placed in running water, is called dialysis and the apparatus used for effecting such separation is called dialyser.
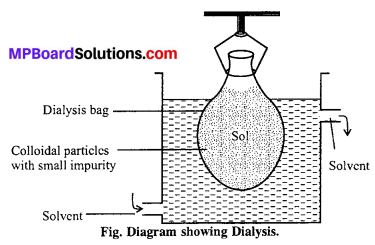
Graham’s dialyser consist [See Fig] of a parchment (or cellophone or cellulose acetate) bag, half immersed in water. The impure colloidal solution (i.e., a mixture of colloidal solution and crystalloids) is placed in the dialyser and the outside water is continuously renewed. The crystalloids slowly pass freely through the membrane into outside water, while pure colloidal solution is left inside the dialyser. This is, however, a very slow process. By allowing the process to continue over a period and pumping fresh water continually into the container, most the ions are eventually removed and the sols get purified. The membrane used is called semipermeable membrane, because it allows selectively the passage of only crystalloids particle and not colloidal particles.
(iv) Tyndall effect: In 1869 Tyndall noted that when a beam of light passes through colloidal solution, in a dark place, path of light is illu¬minated by bluish light. Colloidal particles scatter light and due to this scattering the light path, way is seen like a bright cone.
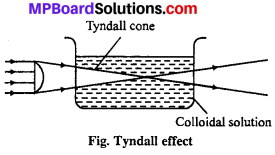
This effect is known as Tyndall effect. The same phenomenon is observed when a beam of sunlight passes through a small slit or hole in a dark room. This cone is perpendicular to light path.
![]()
Question 24.
Give four uses of emulsions.
Answer:
Applications or uses of emulsion :
- Cleaning action of soaps and detergents are based on emulsification.
- Emulsions are used as medicines e.g., codliver oil.
- Many disinfectants like phenyl, dettol, lysol etc., are when mixed with water, form emulsions like oil.
- Froth floatation process for concentration of ore is based on the principle of emulsification.
- Digestion of fat by intestine also involves emulsification.
Question 25.
Explain the terms with suitable examples :
(i) Alcosol
(ii) Aerosol
(iii) Hydrosol.
Answer:
(i) Alcosol: It is a colloidal solution in which alcohol is the dispersion medium. For example, colloids which has cellulose nitrate as dispersed phase and ethyl alcohol as dispersion medium.
(ii) Aerosol: It is a colloidal solution in which liquid is a dispersed phase and gas is a dispersion medium e.g., fog, mist, cloud etc..
(iii) Hydrosol: It is a colloidal solution in which solid is a dispersed phase and water is a dispersion, e.g., gold sol, arsenious sulphide sol, ferric oxide sol etc.
Question 27.
Comment on the statement that “Colloid is not a substance but a state of substance”.
Answer:
The given statement is true. It is because colloidal is not the property of substance. Colloidal is a state in which all the substance can be brought by suitable methods. If the size of the particle lies in the range 1 nm to 1000 nm, it is in the colloidal state.
![]()
Surface Chemistry Other Important Questions and Answers
Surface Chemistry Objective Type Questions
Question 1.
Choose the correct answer :
Question 1.
In the adsorption process of acetic acid on activated charcoal, acetic acid is :
(a) Adsorber
(b) Absorber
(c) Adsorbent
(d) Adsorbate.
Question 2.
Cause of stability of lyophobic sol is :
(a) Brownian movement
(b) Tyndall effect
(c) Electric charge
(d) Brownian movement and Electric charge.
Question 3.
In the coagulation of As2S3 colloidal solution value of whose coagulating power will be minimum:
(a) NaCl
(b) KCl
(c) BaCl2
(d) AlCl3.
Question 4.
Adsorption process is :
(a) Endothermic
(b) Exothermic
(c) No heat change
(d) None of these.
Question 5.
Size of colloidal particles in the range of:
(a) 10-7 to 10-9 cm
(b) 10-9 to 10-1 cm
(c) 10-5 to 10-7 cm
(d) 10-2 to 10-8 cm.
Question 6.
Which of the following is not used for the preparation of lyophilic colloid :
(a) Starch
(b) Gum
(c) Gelatin
(d) Metal sulphide.
Question 7.
Sol which can act like a protective colloid :
(a) As2S3
(b) Gelatin
(c) Au
(d) Fe(OH)3
Question 8.
Fog is an example of colloidal system of:
(a) Liquid dispersed in gas
(b) Gas dispersed in gas
(c) Solid dispersed in gas
(d) Solid dispersed in liquid.
Question 9.
Gelatin is mostly used in making ice creams in order to :
(a) Prevent forming a colloidal sol.
(b) Enrich the fragrance
(c) Prevent crystallization and stabilize the mixture
(d) Modify the taste.
Question 10.
In the reaction
![]()
(a) Auto catalyst
(b) Poison
(c) Negative catalyst
(d) Positive catalyst.
Question 11.
The migration of colloidal particles under the influence of electric fields is :
(a) Cataphoresis
(b) Electrodialysis
(c) Electrophoresis
(d) Electrical dispersion.
Question 12.
Hardy-Schulze law is related with :
(a) Solution
(b) Coagulation
(c) Solids
(d) Gases.
Question 13.
How many phases are present in a colloidal solution :
(a) 1
(b) 2
(c) 3
(d) 4.
Question 14.
Which is an emulsion among the following :
(a) Air
(b)Wood
(c) Butter
(d) Milk.
Question 15.
Butter is:
(a) A gel
(b) An emulsion
(c) A sol
(d) Not a form of colloid.
Question 16.
Sol which acts as protective colloid is :
(a) Gelatin
(b) Au
(c) As2S3
(d) Fe(OH)2
Question 17.
Which is not correct for physisorption :
(a) A reversible process
(b) Needs low heat of adsorption
(c) Needs activation energy
(d) Needs low temperature.
Question 18.
In which among the following is Tyndall effect not expected :
(a) Suspension
(b) Emulsion
(c) Sugar solution
(d) Gold sol.
Answers:
1. (d), 2. (d), 3. (d), 4. (b), 5. (c), 6. (d), 7. (b), 8. (a), 9. (c), 10. (b), 11. (c), 12. (b), 13. (b), 14. (d), 15. (a), 16. (a), 17. (c), 18. (c).
Question 2.
Fill in the blanks :
- Rate of physical adsorption ………………………… with the increase in temperature.
- Particles of As2S3 sol are …………………………
- In the contact process of manufacture of H2SO4 for Pt catalyst ………………………… act as …………………………
- Oxidation of oxalic acid by KMnO4 is an example of …………………………
- Biological catalysts are necessarily …………………………
- Movement of colloidal particles under the effect of electric field is called …………………………
- Scattering of light by colloidal particles is called ………………………… effect|
- Intermediate compound theory is applicable to ………………………… catalyst
- The substance on whose surface adsorption takes place is called an …………………………
- Milk is an example of …………………………
- Blood is a ………………………… charged colloid.
- Adsorption is an ………………………… process.
- Colloidal solution of solid in liquid is called …………………………
- Catalytic promoter substance is …………………………
- On adding electrolyte, precipitation of colloidal particles is known as …………………………
- According to Hardy Schulze law, coagulating capacity of ions depend on the ………………………… of ions
Answers:
- Decreases
- Negatively charged
- As2O3, poison
- Auto catalysis
- Enzyme
- Electrophoresis
- Tyndall effect
- Homogeneous
- Adsorbent
- Emulsion
- Negatively
- Exothermic
- Gel
- Molybdenum
- Coagulation
- Charge.
![]()
Question 3.
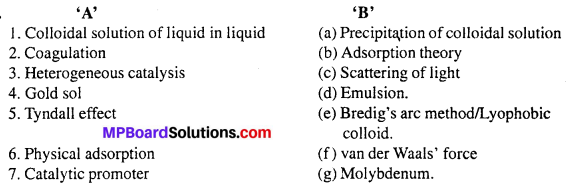
Match the following :
I.

Answers:
- (f)
- (d)
- (a)
- (c)
- (g)
- (e)
- (b).
II.
Answers:
- (d)
- (a)
- (b)
- (e)
- (c)
- (f)
- (g).
Question 4.
Answer in one word/sentence :
- What happen when gelatin is mixed into gold sol ?
- The substance which increases the strength of the catalyst but do not work as catalyst.
- Dissociation of emulsion into its constituent liquid is known as :
- The catalyst which is used for hydrogenation of oils ?
- The decomposition of hydrogen peroxide by phosphoric acid represents which kinds of catalysis ?
- Which catalyst converts glucose into alcohol ?
- Among Na+, Ba+2, Al+3, Sn+4 ions which one has the strongest coagulation power ?
- Cl2 gas mask works on which principle ?
- Conversion of a precipitate into colloidal particles is known as:
- Cleansing action of soap based on which principle ?
- Who used the word catalyst for the first time ?
- Write the size of colloidal particles.
- Write nature of catalyst used in oxidation of oxalic acid by KMnO4.
- Give an example of catalytic poisoning.
- Movement of particles of colloids is called.
- Which one catalyst is used for hydrolysis of cane sugar.
Answer:
- Protection
- Catalyst promotor
- Demulsification
- Ni
- Negative catalyst
- Zymase
- Sn+4
- Adsorption
- Peptization
- Emulsification
- Bergellius
- 10-5 to 10-7 cm
- Auto catalyst
- As2O3 in contact process
- Brownian motion
- Invertase enzyme.
![]()
Surface Chemistry Very Short Answer Type Questions
Question 1.
Which inert gas is adsorbed in (i) Minimum amount and (ii) Maximum amount on the surface of gas charcoal ?
Answer:
- Helium because of its lower atomic mass and the weakest van der Waals’ forces of attraction is adsorbed on gas charcoal in minimum amount.
- Xenon because of its high atomic mass and the strongest van der Waals’ forces of attraction is adsorbed on gas charcoal in maximum amount.
Question 2.
Why is sky blue in colour ?
Answer:
Dust particles present in air forms colloidal solution whose particles shows Tyndall effect and hence sky appears blue in colour.
Question 3.
KMnO4 decolourises slowly initially and then rapidly when added to oxalic acid. Why ?
Answer:
Addition of KMnO4 to oxalic acid oxidises it. Mn+2 ion produced in this reaction acts as autocatalyst and hence decolourisation occur rapidly.
2MnO–4 + 5C2O-24 + 16H– → 2Mn+2 + 10CO2 + 8H2O
Question 4.
What is Colloidal solution ?
Answer:
The heterogeneous solution in which solute particles of 10-7 cm to 10-4 cm diameter and solvent particles are within 10-8 cm to 10-7 cm are called colloidal solution.
e.g. Milk, blood, cloud, smoke, etc.
Question 5.
What is adsorbent ?
Answer:
Solid substances which adsorb the gases or solution are called adsorbent. Like : animal charcoal, silica etc.
Question 6.
What are adsorbate ?
Answer:
Gas molecules, vapours or ions which are adsorbed on the surface of solids are called adsorbate.
Question 7.
Delta is formed when river water meets sea water. Explain.
Answer:
In the river water negatively charged particles of soil and sand are present. When river water meets sea-water, various ions Na+, K+ or Mg++ present in sea water causes coagulation and soil particles etc. are settle down. Thus a delta is formed.
Question 8.
How is rain possible by spraying silver iodide on clouds ?
Answer:
Clouds are charged due to colloidal nature. Silver iodide is an electrolyte. Spray¬ing it on clouds lead to coagulation by which it rains.
Question 9.
How is the adsorption of a gas related to its critical temperature ?
Answer:
Higher is the critical temperature of a gas, greater is the ease of liquification of gas i.e., larger are the van der Waals’ forces of attraction. Therefore, greater is the adsorption.
Question 10.
What happen when a freshly precipitated Fe(OH)3 is shaken with little amount of dilute solution of FeCl3 ?
Answer:
A reddish brown colloidal solution of Fe(OH)3 is obtained. This process is called peptization. The Fe+3 ions from FeCl3 are absorbed on the surface of the precipitate and form positively charged colloidal solution.
Fe(OH)3 + Fe+3 → [Fe(OH)3]Fe+3
![]()
Surface Chemistry Short Answer Type Questions
Question 1.
What is meant by colloidal solution ? What is dialysis ? What is its principle ?
Answer:
Colloidal solution: The solutions in which solute particles are of 10-7 cm. to 10-4 cm. iameter and solvent particles are within 10-8 cm. to 10-7 cm., are called colloidal solution. Colloidal solutions are heterogeneous in nature.
Dialysis : The process by which dissolved impurities are removed with the help of parchment paper or membrane, is called dialysis.
Colloidal solution is filled in a bag, made of parchment paper and suspended in distilled water. Particles of dissolved crystalloids come out of the bag and are diffused in the water. Thus, the colloidal solution is purified.
Question 2.
Define Sol, Gel and Emulsion with example.
Answer:
When the dispersed phase of colloidal solution is solid and dispersion medium is liquid, it is called sol.
e.g., Colloidal solutions of ferric hydroxide, gold etc. in water.
Gel: When any liquid is dispersed in solid and form colloidal solution, is called gel.
e.g., Paneer, butter etc.
Emulsion : When dispersed state and dispersion medium both are liquid, it is called emulsion.
e.g., Milk, Cream etc.
Question 3.
What is homogeneous and heterogeneous catalysis ? Explain with example.
Answer:
Homogeneous catalysis : The chemical reaction in which reactants and catalysts are in same physical state, are example of homogeneous catalysis.
Example : In lead chamber process of manufacturing of sulphuric acid reactant and catalyst NO are in same physical state – gaseous.
![]()
Heterogeneous catalysis : The chemical reactions in which reactants and catalysts are in different physical state, are examples of heterogeneous catalysis.
Example: In Haber process manufacture of Ammonia.
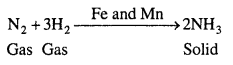
Question 4.
What is ‘Gold number’ ? Explain with example.
Answer:
Gold Number: Protective actions of different lyophilic colloids are compared with gold number. Gold number can be defined as :
“Gold number is the number of miligrams of protective colloid (Lyophilic) which on adding prevents the coagulation of 10 ml. standard gold sol by addition of 1 ml. of 10% NaCl solution.”
Thus smaller the value of gold number, higher is it’s protecting action.
For example, gold number of gelatin and gum arobic are 0.005 and 015 respectively. It means gelatin is better protective colloid because it’s only 0-005 mg quantity is required to prevent coagulation if 10 ml Au sol by addition of 1 ml. 10% NaCl.
Question 5.
What is the importance of emulsifying agents in emulsifications ?
Answer:
The process of making emulsions is called emulsification. Emulsions are formed when suitable liquids are mixed and shaked, but the emulsions are thus formed are not stable. To make these emulsions stable some other substances are added, which are known as emulsifying agents. Soap, gum, starch etc. act as emulsifying agents. In absence of emulsifying agents, disperse drops of liquid meet each other to distroy emulsion state. It is supposed that emulsifying agents form membrane at interphase of oil and water which checks the union of droplets.
Question 6.
What is adsorption ? Give its two example and explain its mechanism.
Answer:
Adsorption is a surface phenomenon in which particles of different substances or gases are temporary linked with the surface of liquid or solids to satisfy the free valencies of solid surfaces.
Examples of adsorption :
(i) In sugar industry, animal charcoal adsorbs coloured materials.
(ii) In permutit process, Ca2+, Mg2+ ions are adsorbed and Na+ ion are released by which hardness of water is removed.
Mechanism of adsorption : Atoms or molecules present on the surface of solid con¬tain free valencies. These free valencies attract the molecules of adsorbate.
Question 7.
What do you understand by Electro-dialysis ?
Answer:
Electro-dialysis : Particles of true solutions pass through parchment paper or cellophane but sol particles cannot pass through such membranes. In dialysis, the sol filled in a bag of parchment or cellophane is suspended in pure water. This process is very slow and takes a long time for completion. However, it can be quickened under the influence of an electric field and the process is known as Electro-dialysis.
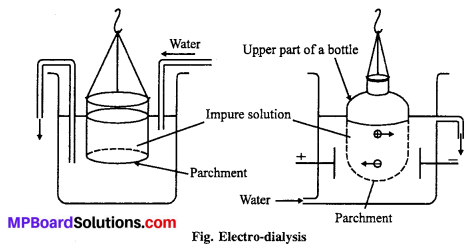
Artificial kidney used in medical science works on the principle of dialysis.
Question 8.
What do you understand by protective colloids ?
Answer:
Protective colloid: On adding small quantity of electrolyte, Lyophobic colloids easily coagulated but if some quantity of hydrophillic colloid is added in Lyophobic colloids, it minimize the effect of addition of electrolyte and coagulation is prevented or very slowly coagulated. This process is called protection of colloid. The hydrophillic colloid which is responsible for protection against coagulation is called protective colloid.
Example: If a small amount of gum, gelatin or starch is added to As2S3 sol, its coagulation of NaCl solution is prevented. Thus starch, gum or gelatin protects the As2S3 sol.
Question 9.
Give the method of preparation of colloidal solution of ferric hydroxide and sulphur in water.
Answer:
(i) To prepare sol of ferric hydroxide, FeCl3 solution is added in the boiling water dropwise with shaking. Excess of FeCl3 and HCl are separated by electrodialysis and sol is also stabilized by this.
FeCl3 + 3H2O → Fe(OH)3 + 3HCl
(ii) H2S gas is passed in the solution of nitric acid (oxidising agent) and colloidal solution of sulphur is obtained.
2HNO3 + H2S → S + 2H2O + 2NO2.
Question 10.
Explain physical and chemical adsorption.
Answer:
In physical adsorption the adsorbate molecules are all reacted by van der Waals’ forces on the surface of adsorbent. These van der Waals’ forces are weak in nature. The physical adsorption is also known as van der Waals’ adsorption or physical adsorption.
In chemical adsorption, the adsorbate molecules are attached with adsorbent through some chemical bonds, because some unsatisfied free valencies exist m the surface of adsorbent. This adsorption is also known as chemisorption.
Question 11.
What do you understand with enthalpy of adsorption ?
Answer:
In adsorption process, when one mole of adsorbate is adsorbed on the surface of adsorbent, the enthalpy change in this is called enthalpy of adsorption or heat of adsorption. The enthalpy of adsorption for chemisorption as about 400 kJ/mol while in physical adsorption it is about 40 kJ/mol.
Question 12.
What is catalysis ? Explain induced catalysis with an example.
Answer:
Catalysis : A catalyst is a substance which alters the rate of chemical reaction without being used in the reaction and the phenomenon is known as catalysis.
Induced Catalysis : In induced catalysis, one reaction already taking place, catalyse the other reaction also which does not occur separately.
Example : Sodium sulphite (Na2SO3) oxidise in the atmosphere easily while sodium arsenite (Na3AsO3) does not oxidise in atmosphere separately. When (Na2SO3) and Na3AsO3 both are kept together, both are oxidised.
Question 13.
Explain positive and negative catalysis with example.
Answer:
Positive Catalysis : When the velocity of any chemical reaction increases with the presence of catalyst, this type of catalysis is called positive catalysis.
![]()
Negative Catalysis : Catalysts when decrease the velocity of chemical reaction are called negative catalysts or retarders and the process is known as negative catalysis.
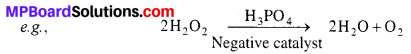
Question 14.
What is peptization ? Explain.
Answer:
Peptization : Peptization is a good method of preparing colloidal sols from precipitates. A fresh precipitate is taken for the purpose and a suitable reason or peptizing agents are added. These peptizing agents are generally dilute solutions of electrolytes of common ion. Peptization process is op-posite of the process of coagulation.
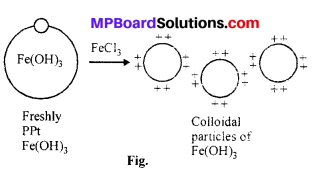
Example : When fresh precipitate of aluminium hydroxide is boiled with dilute HCl mixed water, colloidal solution of Al(OH)3 is obtained.
When an electrolyte is added to a fresh precipitate then the particles of the electrolyte preferentially adsorbs an ion and due to electrostatic repulsion undergo in colloidal state. On adding electrolyte ferric chloride to ferric hydroxide precipitate, sol of ferric hydroxide is obtained.
Question 15.
Deduce the origin of electrical charge on colloidal particles.
Answer:
Colloidal particles have electrical charge and all colloidal particles of any colloidal solution have similar positive or negative charge. To explain origin of charge, following theories are given :
(i) Due to internal friction : According to this theory charge on colloidal particles originates due to mutual friction of dispersion medium and dispersed phase.
(ii) By ionisation of groups present in surface: Colloidal particles may also acquire charge by direct ionisation of different groups present in the surface. These groups may be either colloidal particles or any electrolyte added to prevent coagulation.
(iii) Electrical double layer theory : According to this theory electrical charge on colloidal particles is double layered. One layer is consist of absorbed ions and other layer is diffused layer which is consist of oppositely charged ions in dispersion medium.
Question 16.
Give reason :
(i) Milk turns sour on adding acid to it.
(ii) Alum is added to purify water.
(iii) Delta is formed where river water meets sea.
Answer:
(i) Milk is an emulsion of fats dispersed in water. Albumin and caesin are emulsifiers. Addition of acid destroys emulsifiers and hence milk gets coagulated.
(ii) In impure water, soil particles, bacteria and other soluble impurities are dissolved.
When alum is added, the Al+3 ion present in alum destroys the negative charge of impure water. Due to neutralization of charge the impurities are coagulated and settle down.
(iii) In the river water negatively charged particles of soil and sand are present. When river water meets sea-water, various salts present in sea water causes coagulation and soil particles etc. are settle down. Thus a delta is formed.
Question 17.
Write characteristics of catalysts.
Answer:
Characteristics of catalysts :
- No participation : Catalysts do not participate in the chemical reaction. Only it’s physical state may be changed.
- Small quantity : A small quantity of catalyst is always required for catalysing reaction.
- No effect in equilibrium: Catalysts do not affect equilibrium state because catalyse both forward and backward reactions.
- Specific nature: Catalysts are of specific nature and catalyse only definite reaction not all.
- Effective temperature : There is a optimum temperature in which catalyst’s activity is maximum
- Promotors and Catalytic poisons : There are some substances which increase the activity of catalyst, by their presence. These substances are known as promotors. Catalytic poisons are substances which destroy the catalytic activity.
Question 18.
Coloured glass, smoke, milk, cream, fog, jelly, pumic stone and soap lather belongs to which type of colloidal system? Name them.
Answer:

Question 19.
Compare the properties of true solution, colloidal solution and suspension.
Answer:
Distinction between True solution, Colloidal solution and Suspension :
Question 20.
What do you understand by activation of the adsorbent ? How is it obtained ?
Answer:
Activation of adsorbent means, increasing the activation power of adsorbent. It can be done by increasing the surface area of adsorbent which can be obtained as follows :
- By removing the adsorbed gases or by heating charcoal in vacuum to extremely high temperature in steam at 650K to 1330K temperature.
- By breaking the adsorbate into small pieces.
- By making the surface of adsorbent rough.
Question 21.
What is the role of adsorbent in heterogenous catalysis ?
Answer:
Generally in heterogenous catalysis, adsorbate is in gaseous where as catalyst is in solid state. Adsorbate molecules are adsorbed on the surface of solid catalyst by physical or chemical adsorption. Due to increase in concentration of adsorbed molecules or by the formation of active species due to breaking up of adsorbed molecules due to reaction occurs fast. Product molecules are desorbed and catalytic surface again becomes available for the adsorption of reactant molecules. This principle is known as adsorption of heterogenous catalysis.
![]()
Surface Chemistry Long Answer Type Questions
Question 1.
Explain the following :
(i) Brownian movement
(ii) Autoca-talysis.
Answer:
(i) Brownian movement : It is observed by an ultra-microscope that colloidal particles move in a zig-zag path in all possible directions. Thus, zig-zag or random motion of sol particles is called Brownian movement. It is caused due to unequal bombardment of Small particle the molecules of dispersion medium on the sol particles. First of all this type of movement is reported by Robert Brown so it is known as Brownian movement. On increasing the size of particles, their movements also decreases and in suspension it is finished.
(ii) Autocatalysis: In autocatalysis, one of the product behave as catalyst and increases the velocity of reaction.
Example : Hydrolysis of Ethyl acetate forms Acetic acid which act as autocatalyst.
CH3COOC2H5 + H2O → [CH3COOH] + C2H5OH
Acetic acid
Question 2.
Write differences between lyophilic and lyophobic colloids.
Answer:
Differences between Lyophilic and Lyophobic colloids :
| Property | Lyophilic colloid | Lyophobic colloid |
| 1. Stabilization | Self stabilized. Do not need any electrolyte for stabilization. | Low stability, carry small quantities of electrolytes added for stabilization. |
| 2. Method of preparation | Easily prepared by dissolving in the solvent. | Colloidal solution is prepared by special methods. |
| 3. Nature | Reversible. It can be re converted to the sol state by simply agitating them with the dispersion medium. | Irreversible. Once coagulated cannot be reconverted. |
| 4. Hydration | Particles are heavily hydrated. | Particles are usually poorly hydrated. |
| 5. Viscosity and surface tension | Surface tension generally lower and viscosity much higher than that of water. | Surface tension and viscosity almost equal to that of water. |
Question 3.
Write Hardy Schulze’s law.
Answer:
Hardy-Schulze’s law : Coagulating power of an ion is governed by this law according to which, “Greater the valency of an ion greater will be its coagulating power.”
Thus, to coagulate negative sol, the coagulating power of different cations are found to be in the order :
\(\mathrm{Sn}^{4+}>\mathrm{Al}^{3+}>\mathrm{Ba}^{2+}>\mathrm{Na}^{+}\)
Similarly for the coagulation of a positive sol of Fe(OH)3, the coagulating power of different anions is found to be in the order :
\(\mathrm{PO}_{4}^{3-}>\mathrm{SO}_{4}^{2-}>\mathrm{Cl}^{-}\)
Question 4.
Explain intermediate compound theory of catalysis.
Answer:
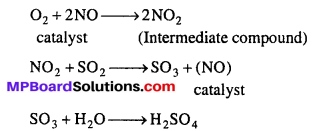
Intermediate compound theory of catalysis : Various theories are given to explain the mechanism of catalysis. This theory explains the mechanism of liquid and gaseous catalysts. According to this theory catalyst combines with one of the reactant and form an intermediate compound. This intermediate compound then reacts with other reactant and form aspected compound and catalyst is released which again acts as catalyst.
For example, if A and B combine and form a compound AB where X is catalyst then AX is intermediate compound.
A + X → AX (intermediatecompound)
AX + B → AB + X
The time in this reaction is less than time required when A and B combine directly.
Example: In lead chamber process of manufacturing of H2SO4, NO acts as catalyst. NO combines with oxygen to form NO2 as intermediate compound. NO2 now reacts with other reagent SO2 and form SO3, the catalyst NO released which further enhance the chain.
Question 5.
Write any five differences between enzyme catalyst and normal catalyst.
Answer:
Differences between enzyme catalyst and normal catalyst:
Enzyme catalyst:
- Is the catalyst for biochemical reactions.
- These are active at normal body temperature and pH.
- These are produced in living cells and are protein in normal form.
- Their molecular mass are high.
- These become inactive at low temperature.
Normal catalyst
- Is the catalyst for normal chemical reactions.
- These function at a definite favourable temperature.
- These can be metals, non-metals or compounds of non-metals.
- Their molecular mass are low. These are also ineffective at low temperature.
Question 6.
What is adsorption ? Explain the factors affecting it
(a) On what factors does the extent of adsorption of gases on solid depend ?
(b) Write five applications of adsorption.
Answer:
Adsorption: Refer Short Answer Type Q. No. 6.
(a) The extent of adsorption of a gas on a solid adsorbent is affected by the following factors:
- Nature of the gas: Under given conditions of temperature and pressure, the easily liquefiable gases like NH3, C02, HC1, etc. are adsorbed to a greater extent than permanent gas.
- Nature of the adsorbent: Activated charcoal is the most common adsorbent for the gases which are easily liquefied.
- Specific area of the solid : Greater the specific area of the solid, greater will be its adsorption power.
- Pressure of the gas : The extent of adsorption increases with increase in pres¬sure.
- Effect of temperature: Adsorption at a surface initially increases till a saturation point is achieved and to this point equilibrium is developed. The magnitude of adsorption decreases with rise in temperature.
- Activation of adsorbent: The adsorption power can be increase by making the surface of adsorbent rough or by breaking it into small pieces. Adsorption power decreases if the particles are made very small because the interparticle space will be two small to allow penetration.
(b) Various applications of adsorption are in our daily life. Some important applications are:
- In Catalysis : In industries various catalysts are used to promote the reaction. These catalysts are generally based on adsorption principle. e.g., Haber process of ammonia, preparation of sulphuric acid by contact process etc.
- In gas masks : Activated charcoal is used to adsorb harmful gases as CO,CH4 etc. Activated charcoal or any other suitable adsorbent are kept in gas masks.
- In creating vacuum : High vacuum can be created by adsorbing gases by adsorbents.
- For decolourising and de-odourising sugar : Animal charcoal is used for decolourising sugar and in de-odourising process.
- In chromatography: Purification of compounds by chromatography is also based on adsorption theory.
Question 7.
Write five applications of colloidal chemistry.
Answer:
The five applications of colloidal chemistry are as follows :
1. In medicines : Colloidal medicines are easily adsorbed by body tissue and are more effective, e.g., Argyrol, colloidal antimony, colloidal gold etc.
2. Smoke precipitation : Smoke and dust are also colloidal in nature and smoke precipitation is based on the principle of electrophoresis. Cottrell precipitator is nowadays commonly used in various industries.
3. Colloids in nature : Different forms of colloidal solutions can be seen in nature. Delta formation, fertile soil, rain, blue colour of sky are some examples of colloidal solutions.
4. Action of Soap: Cleaning action of soap is explained on the basis that soap solution is colloidal. Soap emulsifies greasy and oily materials sticking on the surface of body or clothes.
5. In leather industry: The colloidal particles in hides are positively charged and on soaking the hide in tannin solution (a negative solution) mutual coagulation takes place. This process is called tanning and it makes the hide hard. Solutions of chromium salts are used in place of tannin with advantage and the process is called chrome-tanning.