In this article, we will share MP Board Class 11th Chemistry Solutions Chapter 1 Some Basic Concepts of Chemistry Pdf, These solutions are solved by subject experts from the latest edition books.
MP Board Class 11th Chemistry Solutions Chapter 1 Some Basic Concepts of Chemistry
MP Board Class 11 Chemistry Some Basic Concepts of Chemistry Textbook Questions and Answers
Question 1.
Calculate the molecular mass of the following:
(i) H2O
(ii) CO2
(Hi) CH4.
Solution.
(i) Molecular mass of H2O = 2(1.008 u) + 16.00 u = 18.016 u
(ii) Molecular mass of CO2 = 12.01 u + 2 × 16.00 u = 44.01 u
(iii) Molecular mass of CH2 = 12.01 u + 4(1.008 u) = 16.042 u
Question 2.
Calculate the mass per cent of different elements present in sodium sulphate (Na2S04).
Solution.
Mass % of an element = \(\frac{\text { Mass of that element in the compound }}{\text { Molecular mass of the compound }} \times 100\)
Molecular mass of Na2SO4 = 2(23.0)+32.0+4 × 16.0 = 142 g mol-1
Mass per cent of sodium = \(\frac{46}{142}\) × 100 = 32.39 %
Mass per cent of sulphur = \(\frac{32}{142}\) × 100 = 22.54 %
Mass per cent of oxygen = \(\frac{64}{142}\) × 100 = 45.07 %
Question 3.
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass. (Atomic mass: Fe = 55.85 amu, O = 16.00 amu).
Solution.
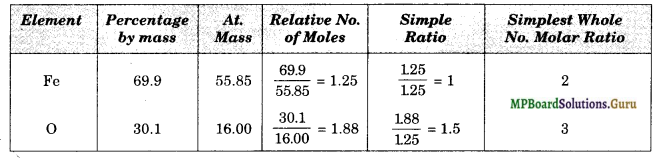
Therefore, the empirical formula = Fe2O3 .
Question 4.
Calculate the amount of carbon dioxide that could be produced when
(i) 1 mole of carbon is burnt in air.
(ii) 1 mole of carbon is burnt in 16 g of dioxygen.
(iii) 2 moles of carbon are burnt in 16 g of dioxygen.
Solution.
The balanced equation for the combustion of carbon in dioxygen or air is
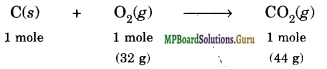
(i) In air, combustion is complete.
Hence, 1 mole of carbon on combustion produces CO2 = 44 g.
(ii) As only 16 g of dioxygen is available, it is the limiting reactant.
Hence, CO2 produced = \(\frac{44}{32}\) × 16 = 22 g.
(iii) Here again, dioxygen is the limiting reactant.
Therefore, CO2 produced from 16 g dioxygen
= \(\frac{44}{32}\) × 16 = 22 g.
Question 5.
Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol-1.
Solution.
0.375 M aqueous solution means that 1000 mL of the solution contains sodium acetate = 0.375 mole
∴ 500 mL of the solution should contain sodium acetate
= \(\frac{0.375}{2}\) mole = \(\frac{0.375}{2}\) × 82.0245 g
Hence, mass of sodium acetate required
= \(\frac{0.375}{2}\) mole × 82.0245 g mol-1
= 15.380 g.
![]()
Question 6.
Calculate the concentration of nitric acid in moles per litre in a sample which has a density, 1.41 g mL-1 and mass per cent of nitric acid in it being 69%.
Solution.
Mass per cent of 69% means that 100 g of nitric acid solution contains 69 g of nitric acid.
Molar mass of nitric acid (HNO3) = 1 + 14 + 48 = 63 g mol-1
∴ Moles in 69 g HNO3 = \(\frac{69 \mathrm{~g}}{63 \mathrm{~g} \mathrm{~mol}^{-1}}\) = 1.095 mole
Volume of 100 g nitric acid solution = \(\frac{100 \mathrm{~g}}{1.41 \mathrm{~g} \mathrm{~mL}^{-1}}\) = 70.92 mL = 0.07092 L
∴ Cone, of HNO3 in moles per litre = \(\frac{1.095 \mathrm{~mole}}{0.07092 \mathrm{~L}}\) = 15.44 M.
Alternatively, (shortcut)
Molarity = \(\frac{\text { Mass per cent } \times \text { density } \times 10}{\text { Molecular mass }}\)
= \(\frac{69 \times 1.41 \times 10}{63}\)
= 15.44 M.
Question 7.
How much copper can be obtained from 100 g of copper sulphate (CuSO4)?
(Atomic mass of Cu = 63.5 u).
Solution.
Molar mass of CuS04 = 63.5 + 32 + 4 × 16 = 159.5 g mol-1
![]()
Thus, 159.5 g of CuSO4 shall give Cu = 63.5 g
Hence, Cu that can be obtained from 100 g of CuSO4 = \(\frac{63.5}{159.5}\) × 100
= 39.81 g.
Question 8.
Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively. Given that the molar mass of the oxide is 159.8 g mol-1 (Atomic mass: Fe = 55.85, O = 16.00 u).
Solution.
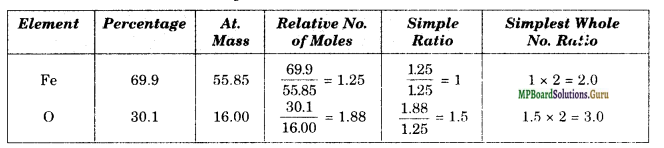
Therefore, the empirical formula = Fe2O3.
Empirical formula mass of Fe2O3 = 2 × 55.85 + 3 × 16.00 = 159.7 g mol-1
Molecular formula = n × Empirical formula
∴ n = \(\frac{\text { Molar mass }}{\text { Empirical formula mass }}\) = \(\frac{159.8}{159.7}\) = 1
Hence, molecular formula is same as empirical formula, viz., Fe2O3.
Question 9.
Calculate the atomic mass (average) of chlorine using the following data:

Solution.
Average atomic mass = \(\frac{\text { R. } A(1) \times \text { At. }{mass}(1)+R . A(2) \times \text { At. mass }(2)}{\text { R.A (1) + R.A (2) }}\) (R.A. = Relative abundance)
Average atomic mass = (0.7577) (34.9689 u) + (0.2423) (36.9659 u) = 26.4959 + 8.9568 = 35.4527.
Question 10.
In three moles of ethane (C2H6), calculate the following:
(i) Number of moles of carbon atoms
(ii) Number of moles of hydrogen atoms
(iii) Number of molecules of ethane.
Solution.
(i) 1 mole of C2H6 contains 2 moles of carbon atoms
∴ 3 moles of C2H6 will contain C-atoms = 6 moles
(ii) 1 mole of C2H6 contains 6 moles of hydrogen atoms
∴ 3 moles of C2H6 will contain H-atoms = 18 moles
(iii) 1 mole of C2H6 contains Avogadro’s number, i.e., 6.02 × 1023 molecules
∴ 3 moles of C2H6 will contain ethane molecules
= 3 × 6.02 × 1023
= 18.06 × 1023 molecules.
![]()
Question 11.
What is the concentration of sugar (C12H22O11) in mol L-1 if its 20 g are dissolved in enough water to make a final volume up to 2 L?
Solution.
Molar mass of sugar (C12H22O11)
= 12 × 12 + 22 × 1 + 11 × 16 = 342 g mol-1
Number of moles in 20 g of sugar = \(\frac{20 \mathrm{~g}}{342 \mathrm{~g} \mathrm{~mol}^{-1}}\) = 0.0585 mole
Molar concentration = \(\frac{\text { Moles of solute }}{\text { Volume of sol. in L }}\) = \(\frac{0.0585}{2}\)
= 0.0293 M.
Question 12.
If the density of methanol is 0.793 kg L-1, what is its volume needed for making 2.5 L of its 0.25 M solution ?
Solution.
Molar mass of methanol (CH3OH)
= 32 g mol-1 = 0.032 kg mol-1
1 litre of 0.25 M CH3OH contains moles of CH3OH = 0.25
∴ 2.5 L of 0.25 M CH3OH will contain moles = 0.25 × 2.5 = 0.625
Mass of 0.625 moles of CH3OH = 0.625 × 32 = 20 g
The density of CH3OH is 0.793 kgL-1 or 0.793 g cm-3
Hence, volume of methanol required volume = \(\frac{\text { mass }}{\text { density }}\)
= \(\frac{20 \mathrm{~g}}{0.793 \mathrm{~g} \mathrm{~cm}^{-3}}\)
= 25.22 mL.
Question 13.
Pressure is determined as force per unit area of the surface. The S.I. unit of pressure, pascal, is as shown below: ,
1 Pa = 1 Nm-2 .
If mass of air at sea level is 1034 g cm-2, calculate the pressure in pascal. Solution. Pressure is the force (i.e., weight) acting per unit area But weight (W) = mg
∴ Pressure = Weight per unit area = \(\frac{1034 \mathrm{~g} \times 9.8 \mathrm{~m} \mathrm{~s}^{-2}}{\mathrm{~cm}^{2}}\)
= \(\frac{1034 \mathrm{~g} \times 9.8 \mathrm{~m} \mathrm{~s}^{-2}}{\mathrm{~cm}^{-2}} \times \frac{1 \mathrm{~kg}}{1000 \mathrm{~g}} \times \frac{100 \mathrm{~cm} \times 100 \mathrm{~cm}}{1 \mathrm{~m} \times 1 \mathrm{~m}} \times \frac{1 \mathrm{~N}}{\mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-2}} \times \frac{1 \mathrm{~Pa}}{1 \mathrm{Nm}^{-2}}\)
= 1.01332 × 105 Pa
Question 14.
What is the S.I. unit of mass? How is it defined?
Solution.
S.I. unit of mass is kilogram (kg). For definition, Mass of a substance is the amount of matter present in it.
Question 15.
Match the following prefixes with their multiples :
Prefixes Multiplies
(i) micro 106
(ii) deca 109
(iii) mega 10-6
(iv) giga 10-15
(v) femto 10
Solution.
micro = 10-6, deca = 10, mega = 10-6, giga = 109, femto = 10-15.
Question 16.
What do you mean by significant figure?
Solution.
The number of significant figures in a measured quantity is equal to the number of digits whose values are known with certainty, plus the first uncertain digit.
Question 17.
A sample of drinking water was found to be severely contaminated with chlo¬roform, CHCl3, supposed to be carcinogen. The level of contamination was 15 ppm (by mass).
(i) Express this in per cent by mass.
(ii) Determine the molality of chloroform in the water sample.
Solution.
(i) 15 ppm means 15 parts in million (106) parts
∴ % by mass = \(\frac{15}{10^{6}}\) x 100
= 15 × 10-4
= 1.5 × 10-3%
(ii) Molar mass of chloroform (CHCl3)
= 12 + 1 + 3 × 25.5
= 119.5 g mol-1
100 g of the sample contain chloroform = 1.5 × 10-3 g
∴ 1000 g (1 kg) of the sample will contain chloroform = \(\frac{1.5 \times 10^{-3}}{100}\) = 1.5 × 10-2 g
Moles of chloroform in 1 kg (m)
= \(\frac{1.5 \times 10^{-2}}{119.5}\) = 1.255 × 10-4
∴ Molality = 1.255 × 10-4m.
Question 18.
Express the following in scientific notation:
(i) 0.0048
(ii) 234,000
(iii) 8008
(iv) 500.0
(v) 6.0012.
Solution.
(i) 4.8 × 10-3
(ii) 2.34 × 105
(iii) 8.008 × 103
(iv) 5.000 × 102
(v) 6.0012 × 10°
Question 19.
How many significant figures are present in the following?
(i) 0.0025
(ii) 208
(iii) 5005
(iv) 126,000
(v) 500.0
(vi) 2.0034
Solution.
(i) 2
(ii) 3
(iii) 4
(iv) 3
(v) 4
(vi) 5.
![]()
Question 20.
Round up the following up to three significant figures:
(i) 34.216
(ii) 10.4107
(iii) 0.04597
(iv) 2808
Solution.
(i) 34.2
(ii) 10.4
(iii) 0.0460
(iv) 2810.
Question 21.
The following data were obtained when dinitrogen and dioxygen react together to form different compounds:
(i) 14 g 16 g
(ii) 14 g 32 g
(iii) 28 g 32 g
(iv) 28 g 80 g
(a) Which law of chemical combination is obeyed data? Give its statement.
(b) Fill in the blanks in the following conversions:
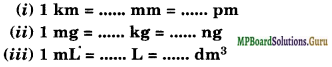
Solution.
(a) Taking the fixed mass of dinitrogen as 28 g the mass of dioxygen combined will be 32, 64, 32 and 80 g in the given four oxides. These are in the ratio 2: 4: 2: 5 which is a simple whole-number ratio. Hence, the given data obeys the law of multiple proportions.
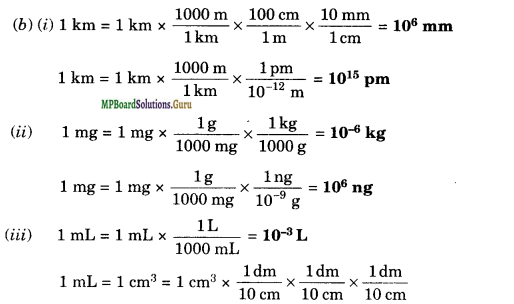
= 10-3 dm3
Question 22.
If the speed of light is 3.0 × 108 ms-1, calculate the distance covered by light in 2.00 ns.
Solution.
Distance covered = Speed × Time
= 3.0 × 108 ms-1 × 2.00 ns
= 3.0 × 108 ms-1 × 2.00 ns × \(\frac{10^{-9} \mathrm{~s}}{1 \mathrm{~ns}}\) = 6.00 × 10-1 m
= 0.600 m
Question 23.
In the reaction, A + B2→ AB2, identify the limiting reagent, if any, in the following mixtures:
(i) 300 atoms of A + 200 molecules of B
(ii) 2 mol A + 3 mol B
(iii) 100 atoms of A + 100 molecules of B
(iv) 5 mol A + 2.5 mol B
(v) 2.5 mol A + 5 mol B.
Solution.
(i) According to the given reaction, 1 atom of A reacts with 1 molecule of B.
∴200 molecules of B will react with 200 atoms of A and 100 atoms of A will be left unreacted. Hence, B is the limiting reagent while A is the excess reagent.
(ii) According to the given reaction, 1 mol of A reacts with 1 mol of B
∴ 2 mol of A will react with 2 mol of B. Hence, A is the limiting reactant.
(iii) No limiting reagent.
(iv) 2.5 mol of B will react with 2.5 mol A as per the given equation. Hence, B is the
limiting reagent.
(v) 2.5 mol of A will react with 2.5 mol of B. Hence, A is the limiting reagent.
Question 24.
Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation:
N2(g) + 3 H2 (g) → 2 NH3(g)
(i) Calculate the mass of ammonia produced if 2.00 × 103 g dinitrogen reacts with 1.00 × 103 g dihydrogen.
(ii) Will any of the two reactants remain unreacted?
(iii) If yes, which one and what would be its mass?
Solution.
(i) According to given equation, 28 g of N2 reacts with 3 mol of H2, i.e., 6 g of H2
∴ 2000 g of N2 will react with H2 = \(\frac{6}{28}\) × 2000 g
= 428.6 g.
Amount of H2 given = 1000 g
Thus, N2 is the limiting reagent while H2 is the excess reagent.
As 28 g of N2 produce NH3 = 2 × 17 = 34 g
∴ 2000 g of N2 will produce NH3 = \(\frac{34}{28}\) × 2000 g
= 2428.57 g
(ii) H2 will remain unreacted.
(iii) Mass of H2 left unreacted = 1000 g – 428.6 g = 571.4 g.
Question 25.
How are 0.50 mol Na2CO3 and 0.50 M Na2CO3 different?
Solution.
Molar mass of Na2CO3 = 2 × 23 + 12 + 3 × 16 = 106 g mol-1
0.50 mol Na2CO3 means 0.50 × 106 g = 53 g
0.50 M Na2CO3 means 0.50 mol, i.e., 53 g Na2CO3 are present in 1 litre of the solution.
Question 26.
If ten volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many volumes of water vapour could be produced?
Solution.
H2 and O2 react according to the balanced equation given as:
2H2(g) + O2(g) → 2H2O(g)
From the equation it is clear that, 2 volumes of H2 react with 1 volume of O2 to produce 2 volumes of water vapour.
Hence, 10 volumes of H2 will react completely with 5 volumes of O2 to produce 10 volumes of water vapour.
![]()
Question 27.
Convert the following into basic units:
(i) 28.7 pm
(ii) 15.15 µs
(iii) 25365 mg
Solution.
(i) 28.7 pm = 28.7 pm × \(\frac{10^{-12} \mathrm{~m}}{1 \mathrm{pm}}\)
= 2.87 × 10-11 m
(ii) 15.15 µs = 15.15 µs × \(\frac{10^{-6} \mathrm{~s}}{1 \mu \mathrm{s}}\)
= 1.515 × 10-5 s
(iii) 2536a mg = 2o365 mg × \(\frac{1 \mathrm{~g}}{1000 \mathrm{mg}}\) × \(\frac{1 \mathrm{~kg}}{1000 \mathrm{~g}}\)
= 2.5365 × 10-2 kg
Question 28.
Which one of the following will have largest number of atoms?
(i) 1 g Au (s)
(ii) 1 g Na (s)
(iii) 1 g Li (s)
(iv) 1 g of Cl2(g)
(Atomic masses: Au = 197, Na = 23, Li = 7, Cl = 35.5 u)
Solution.
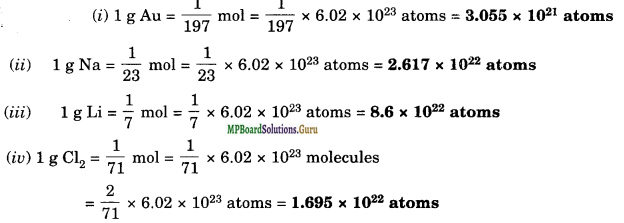
Thus, 1 g of Li has the largest number of atoms.
Question 29.
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040.
Solution.
Given mole fraction of ethanol (XEt0H) = 0.04 i.e.,
XEt0H = \(\frac{n_{\mathrm{EtOH}}}{n_{\mathrm{EtOH}}+n_{\mathrm{H}_{2} \mathrm{O}}}\)
As molality of solution is to be calculated, therefore amount of water to be taken
1 kg = 1000 g
nH2O = \(\frac{1000}{18}\) = 55.55 moles
Hence, 0.04 = \(\frac{n_{\mathrm{EtOH}}}{n_{\mathrm{EtOH}}+55.55}\)
or n<sub>EtOH</sub> = 0.04 × n<sub>EtOH</sub> + 55.55 × 0.04
or n<sub>EtOH</sub> – 0.04 n<sub>EtOH</sub> = 2.222
or 0.96 n<sub>EtOH</sub> = 2.222
Hence, n<sub>EtOH</sub> = \(\frac{2.222}{0.96}\)
= 2.314
Question 30.
What will be the mass of one 12C atom in g?
Solution.
1 mol of 12C atoms = 6.022 × 1023 atoms has mass = 12 g
∴ 1 atom of 12C will have mass = \(\frac{12}{6.022 \times 10^{23}} \mathrm{~g}\) = 1.99 × 10-23 g
Question 31.
How many significant figures should be present in the answer of the following calculations?
(i) \(\frac{0.02856 \times 298.15 \times 0.112}{0.5785}\)
(ii) 5 × 5.364
(iii) 0.0125 +0.7864+0.0215
Solution.
(i) The least precise term has 3 significant figures (;i.e in 0.112). Hence, the answer should have 3 significant figures.
(ii) Leaving the exact number (5), the second term has 4 significant figures. Hence, the answer should have 4 significant figures.
(iii) In the given addition, the least number of decimal places in the term is 4. Hence, the answer should have 4 significant.
Question 32.
Use the data given in the following table to calculate the molar mass of natu¬rally occurring argon.

Solution. Molar mass of Ar = 35.96755 × 0.00337 + 37.96272 × 0.00063 + 39.96924 × 0.99600
= 39.948 g mol-1.
Question 33.
Calculate the number of atoms in each of the following:
(i) 52 moles of He
(ii) 52 u of He
(iii) 52 g of He
Solution.
(i) 1 mol of He = 6.022 × 1023 atoms
∴ 52 mol of He = 52 × 6.022 × 1023 atoms
= 3.13 × 1025 atoms
(ii) 1 atom of He has a mass = 4 u
∴ 52 u of He has number of atoms = \(\frac{1}{4}\) × 52 atoms = 13 atoms
(iii) 1 mole of He has a mass = 4 g = 6.022 ×1023 atoms ,
∴ 52 g of He will contain atoms= \(\frac{6.022 \times 10^{23}}{4}\) × 52 atoms
= 7.8286 × 1024 atoms.
Question 34.
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at S.T.P.) of this welding gas is found to weigh 11.6 g. Calculate
(i) empirical formula
(ii) molar mass of the gas, and
(iii) molecular formula.
Solution.
Mass of carbon in 3.38 g CO2
= \(\frac{12}{44}\) × 3.38 g = 0.9218 g
Mass of hydrogen in 0.690 g H2O
= \(\frac{2}{18}\) × 0.690 g = 0.0767 g
As compound contains only C and H, therefore, total mass of the compound
= 0.9218 + 0.0767 g = 0.9985 g
% of C in the compound = \(\frac{0.9218}{0.9985}\) × 100 = 92.32
% of H in the compound = \(\frac{0.0767}{0.9985}\) × 100 = 7.68
Calculation of Empirical Formula
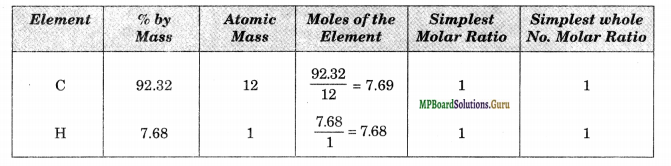
Therefore, the empirical formula = CH
Calculation of Molar Mass
10.0 L of the gas at S.T.P. weigh = 11.6 g
∴ 22.4 L of the gas at S.T.P. will weigh = \(\frac{11.6}{10.0}\) × 22.4 = 25.984 g
Thus, molar mass = 25.98 g mol-1
Empirical formula mass of CH = 12 + 1 = 13
Calculation of Molecular Formula
Molecular mass 25.98
∴ n =\(\frac{\text { Molecular mass }}{\text { E.F. mass }}\) =\(\frac{25.98}{13}\) = 1.998 = 2.0
∴ Molecular formula = (CH)2
= C2H2
Question 35.
Calcium carbonate reacts with aqueous HC1 according to the reaction
CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + CO2 (g) + H2O (l).
What mass of CaCO3 is required to react completely with 25 ml, of 0.75 M HCl?
Solution.
Step 1. To calculate mass of HCl in 25 mL of 0.75 M HCl
1000 mL of 0.75 M HCl contain HCl
= 0.75 mol = 0.75 × 36.5 g
= 27.375 g
∴ 25 mL of 0.75 HC1 will contain HCl
= \(\frac{27.375}{1000}\) × 25 g
= 0.6844 g.
![]()
Step 2. To calculate mass of CaCO3 reacting completely with 0.6844 g of HCl
73 g HC1 react completely with CaCO3 = 100 g
∴ 0.6844 g HCl will react completely with CaCO3
= \(\frac{100}{73}\) × 0.6844 g 73
= 0.9375 g.
Question 36.
Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction.
4 HCl (aq) + MnO2(s)→ 2 H2O(l) + MnCl2 (aq) + Cl2 (g)
How many grams of HC1 react with 5.0 g of manganese dioxide? (Atomic mass of Mn = 55 u).
Solution.
1 mol of MnO2, i.e., 55 + 32 = 87 g, 1 mole of HCl = 36.5 g.
∴ 87 g of MnO2, react HCl = 4 × 36.5
∴ 5.0 g of Mn02 will react with HCl = \(\frac{4 \times 36.5}{87}\) × 5.0 g
= 8.39 g.
MP Board Class 11 Chemistry Some Basic Concepts of Chemistry Important Questions and Answers
Question 1.
Name the disease for which Cisplatin is an effective drug.
Answer:
Cancer.
Question 2.
Name the disease for which taxol is used.
Answer:
Cancer.
Question 3.
Name the drug used for helping AIDS victims.
Answer:
AZT (Azidothymidine).
Question 4.
Which refrigerant is responsible for ozone depletion in stratosphere?
Answer:
CFC (Chloro Fluoro Carbons).
Question 5.
What is a unit?
Answer:
A unit may be defined as the standard of reference choose to measure or express any quantity.
Question 6.
What is meant by fundamental quantities?
Answer:
All physical quantities may be expressed in terms of selected basic quantities called fundamental quantities.
Question 7.
What is derived unit?
Answer:
Any unit obtained by multiplying or dividing one fundamental unit by another fundamental unit is known as derived unit. e.g., unit of area.
Question 8.
Define molar mass.
Answer:
Molar mass is the mass of 1 mole particles of the substance.
Question 9.
What is relative molecular mass?
Answer:
The relative molecular mass of a substance is the sum of relative atomic masses of the constituent atoms present in a molecule of the substance.
Question 10.
Define number of significant figures.
Answer:
The number of significant figures in a measured quantity is the number of digits whose values are known plus the first digit of uncertain values.
![]()
Question 11.
What do you mean by rounding off a number?
Answer:
Rounding off a number means that figures that are not significant for the purpose at hard are dropped.
Question 12.
Define the term precision and accuracy.
Answer:
Precision refers to the closeness of the set of values obtained for identical measurements of a quantity.
Accuracy refers to the closeness of a single measurement to its true value.
Question 13.
How is mole related to volume of gases?
Answer:
1 mole = 22.4 L of gas at N.T.P.
Question 14.
What is a molecule?
Answer:
Molecule is an unchained collection of two or more atoms joined by bond e.g., O2, Cl2, CO2 etc.
Question 15.
What is a homogeneous mixture?
Answer:
A homogenous mixture is a mixture that has same composition throughout. Its components are indistinguishable, e.g., a gaseous mixture, a liquid solution.
Question 16.
Give one example each of
(i) Solid homogeneous mixture
(ii) Gaseous homogeneous solution
Answer:
(i) An alloy e.g., brass, bronze etc.
(ii) Air
Question 17.
State and explain the law of constant composition.
Answer:
Law of constant composition states that a chemical compound is always found to be made up of the same elements combined together in the same ratio by weight, e.g., the ratio of hydrogen and oxygen in pure water is always 1: 8 by weight.
Question 18.
State the law of multiple proportion.
Answer:
Law of multiple proportions states that if two elements A and B combine to give two or more compounds, then the weights of A which combines with fixed weight of B, bear a simple ratio to one another.
Question 19.
State the Gay-Lussac’s law of combining volumes.
Answer:
Gay-Lussac’s law of combining volumes states when gases react together they do so in volumes that bear a simple ratio to one another and the volumes of products (if gaseous), all volumes being measured under similar conditions of temperature and pressure.
Question 20.
State Avogadro’s law.
Answer:
Avogadro’s law states “equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules”.
Question 21.
Define mole.
Answer:
A mole is the amount of substance that contains as many entities (atoms, molecules or other particles) as there are atoms in exactly 0.012 kg (or 12 g) or carbon-12 isotope.
Question 22.
What is Avogadro’s number?
Answer:
Avogadro’s number (NA) is equal to 6.023 × 1023 particles.
Question 23.
Define one atomic mass unit (amu).
Answer:
Atomic mass unit (amu) is defined as \(\frac{1}{12}\) th of the mass of 12C isotope.
Question 24.
What do you mean by fractional abundance of an isotope?
Answer:
Fractional abundance of an isotope is the fraction of the total number of atoms that is composed of a particular isotope.
Question 25.
What is meant by atomicity of a gas?
Answer:
Atomicity is the number of atoms present in one molecule of a gas.
Question 26.
Write the units of molarity.
Answer:
Mol L-1.
Question 27.
What is meant by molarity of a solution?
Answer:
The molarity (M) of a solute in solution is defined as the number of moles of a solute present in one litre of solution.
Molarity =\(\frac{\text { Moles of solute }}{\text { Litres of solution }}\).
![]()
Question 28.
How many atoms are present in one gram-atom of an element?
Answer:
6.023 × 1023 atoms.
Question 29.
Define molecular formula.
Answer:
Molecular formula is that formula of a compound that gives the actual number of atoms of various elements present in a molecule of the compound.
Question 30.
What is limiting reagent?
Answer:
Limiting reagent is that reagent which is completely consumed during the reaction.
Question 31.
What do you mean by chemical stoichiometry?
Answer:
It is that area of chemistry and chemical technology on which the determination of the quantities of reactants or products of a chemical reaction is based.
Question 32.
What are isotopes?
Answer:
Isotopes are atoms of the same element which have different mass number. Isotopes of an element are having some chemical properties but different physical properties.
Question 33.
Write the uses of isotopes.
Answer:
Uses of isotopes are:
(i) Isotope of uranium is used as fuel in nuclear reactors.
(ii) Isotope of cobalt is used in treatment of cancer.
(iv) Isotope of iodine is used in treatment of goitre.