MP Board Class 7th Science Solutions Chapter 6 Physical and Chemical Changes
Activities
Activity 1
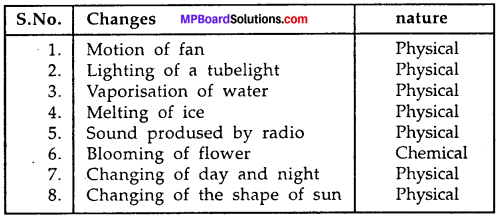
Make a list of eight changes you have noticed from your surroundings.
Answer:
The changes are as:
- Motion of fan
- Lighting of a tubelight
- Vaporisation of water
- Melting of ice
- Sound produced by radio
- Blooming of flower
- Changing of day and night
- Changing of the shape of sun.
![]()
Activity 2
Try to indentify changes that you observe around you as physical or chemical changes.
Answer:
Physical and Chemical Changes Text book Exercises
Question 1.
Classify the changes involved in the following processes as physical or chemical changes?
- Photosynthesis
- Dissolving sugar in water
- Burning of coal
- Melting of wax
- Beating aluminium to make aluminium foil
- Digestion of food
Answer:
- Chemical change
- Physical change
- Chemical change
- Physical change
- Physical change
- Chemical change.
![]()
Question 2.
State whether the following statements are true or false. In case a statement is false, write the corrected statement in you notebook?
- Cutting a log food into pieces is a chemical change. (T/F)
- Formation are from leaves is a physical change. (T/F)
- Iron pipes coated with zinc do not get rusted easily. (T/F)
- Iron and rust are the same substances. (T/F)
- Condensation of steam is not a chemical change. (T/F)
Answer:
- False (F)
- False (F)
- True (T)
- True (T)
- True (T)
Question 3.
Fill in the blanks in the following statements:
- When carbon dioxide is passed through lime water, it turns milky due to the formation of ……………
- The chemical name of baking soda is ……………
- Two methods by which rusting of iron can be prevented are …………… and ……………
- Changes in which only …………… properties of a substance change are called physical changes.
- Changes in which new substances are formed are called …………… changes.
Answer:
- calcium carbonate
- Sodium hydrogen carbonate
- coating, galvanization
- physical
- chemical
Question 4.
When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of changes is it? Explain.
Answer:
It is a chemical change. When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas carbaon – dioxide.
Lemon juice + Baking soda Carbon dioxide + Lime water.
![]()
Question 5.
When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place?
Answer:
Melting of wax is a physical change while burning of candle is a chemical change. Lightning torch bulb using dry cell is another example where both physical and chemical changes takes place. The lighting of the bulb is physical change while current from the dry cell is obtained by the chemical substances inside it.
This is chemical change because the chemicals in the cell get converted into new substances and hence the cell ultimately becomes useless.
Question 6.
How would you show that setting of curd is a chemical change?
Answer:
The conversion of milk into curd, i.e., setting of curd is a permanent as well as irreversible and lead to the production of a new substance. The curd is not be converted into milk. Thus, the formation of curd is a chemical change.
![]()
Question 7.
Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Answer:
Burning of a wood is a chemical change because in addition to new products burning is always accompanied by production of heat. Cutting of wood into small pieces is a physical change because pieces of wood under went changes in size and no new substances is formed.
Question 8.
Describe how crystals of copper sulphate are prepared?
Answer:
Take a cupful of water in a beaker and add a few drops of dilute sulphuric acid. Heat the water. When it starts boiling add copper sulphate powder slowly while stirring continuiously (fig.). Continue adding copper sulphate powder, till no more powder can be dissolved. Filter the solution. Allow it to cool.
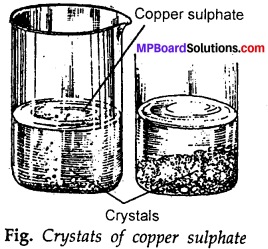
Do not disturb the solution when it is cooling. Look at the solution after some time. Can you see the crystals of copper sulphate? If not, wait for some more time. Crystals of copper sulphate slowly from at the bottom of the beaker.
Question 9.
Explain how painting of an iron gate prevents it from rusting?
Answer:
For rusting, iron must be in contact with both air and moisture. When iron gate is painteel the layer of paint cuts the contact between air, moisture and iron. Thus, it prevents rusting.
![]()
Question 10.
Explain why rusting of iron objects is faster in coastal areas than in deserts?
Answer:
The water of coastal areas containing many salts. The salt water makes the process of rust formation faster. Thus, rusting of iron objects is faster in coastal areas than deserts.
Question 11.
The gas we use in the kitchen is called liquified petroleum gas (LPG). In the cylinder it exist as a liquid. When it comes out from the cylinder it becomes a gas (Change A) then it burns (Change B). The following statements pertain to these changes. Choose the correct one.
- Process – A is a chemical change.
- Process – B is a chemical change.
- Both processes – A and B are chemical changes.
- None of these processes is a chemical change.
Answer:
3. Both processes – A and B are chemical changes.
Question 12.
Anaerobic bacteria digest animal waste and produce biogas (Change A). The biogas is then burnt as fuel (Change B). The following statements pertain to these changes. Choose the correct one.
- Process – A is a chemical change.
- Process – B is a chemical change.
- Both processes – A and B are chemical changes.
- None of these processes is a chemical change.
Answer:
3. Both processes – A and B are chemical changes.
Extended Learning – Activities and Projects
Question 1.
Describe two changes that are harmful. Explain why you consider them harmful. How can you prevent them?
Answer:
1. Spoilage of Food:
Food items when kept carelessly, get spoiled. This is a chemical change and obviously harmful for our health. Basically, the food is spoiled by microorganisms.
Preventation of Food Spoilage:
Microorganisms do not survive high or low temperature. So, food items stored in refrigerator do not spoil. Also, we should keep them covered, so that microorganism do not get any change to spoil them.
2. Rusting:
If a piece of iron is left in open for some time, it acquires a film of brownish substance. This substance is called rust and the process is called rusting.
Iron benches kept in parks, gardens and lawns, iron gates of parks, farm houses, houses; almost every article of iron, kept in open gets rusted.
The process of rusting can be represented by the following equation:
Iron (Fe) + Oxygen (O2, from the air) + water (H2O) → Rust (ironoxide, Fe2O3)
For rusting, the presence of both water (or water vapour) and oxygen is essential. Rusting is harmful because, it destorys the iron objects. Iron is the most widely used metal and so rusting is a serious problem.
Preventation of rusting:
It can be prevented by preventing iron things from coming in contact with water, oxygen, or both. One simple way is to apply a coat of paint or grease. In fact, these coats should be applied regularly to prevent rusting.
Another way is to deposit a layer of a metal like chromium or zinc on iron. This process is called galvanisation. Generally the iron pipes, which are used in our homes for water supply are galvanised to prevent rusting.
![]()
Question 2.
Take three glass bottles with wide mouths. Label them, A, B and C. Fill about half of bottle A with ordinary tap water. Fill bottle B with water which has been boiled for several minutes, to the same level as in A. In bottle C, take the same boiled water and of the same amount as in other bottles. In each bottle put a few similar iron nails so that they are completely under water. Add a teaspoonful of cooking oil to the water in bottle C so that it forms a film on its surface. Put the bottles away for a few days. Take out nails from each bottle and observe them. Explain your observations?
Answer:
The nails in bottle B rust a little, nails in bottle A are the most rusted and that is bottle C remain unchanged. For rusting both oxygen and water are necessary. Both of these factors are present in the bottle A, since oxygen is dissolved in water. In bottle B, water is boiled and hence dissolved air is removed. Due to lack of oxygen, iron nail does not rust much. In bottle C, the layer of oil present dissolution of air in the water and hence no rusting occurs.
Question 3.
Prepare crystals of alum.
Answer:
Take a cupful of water in a beaker and add a few drops of dilute sulphuric acid. Heat the water. When it starts boiling add copper sulphate powder slowly while stirring continuiously (fig.). Continue adding copper sulphate powder, till no more powder can be dissolved. Filter the solution. Allow it to cool.

Do not disturb the solution when it is cooling. Look at the solution after some time. Can you see the crystals of copper sulphate? If not, wait for some more time. Crystals of copper sulphate slowly from at the bottom of the beaker.
Question 4.
Collect information about the types of fuels used for cooking in you area. Discuss with your teachers/ parents/ others which fuels are less polluting and why?
Answer:
The different fuels used for cooking are wood, cow – dung cake, kerosene, biogas and LPG. Among all of these, biogas and LPG are least polluting. Both of these burn completely and do not give smoke. Also there is no residue i.e. ash after burning. Thus, these fuels are less polluting.
Physical and Chemical Changes Additional Important Questions
Objective Type Questions
Question 1.
Choose the correct alternative
Question (i)
Which of the following is not a chemical change –
(a) Digestion of food
(t) Burning of oaal
(c) Curdling of milk
(d) Melting Of ice.
Answer:
(d) Melting Of ice.
Question (ii)
Properties such as shape, size, colour and state of a sub-stance are called its –
(a) Chemical properties
(b) Physical properties
(c) Both (a) and (b)
(d) None of these.
Answer:
(b) Physical properties
![]()
Question (iii)
The substances formed as a result of chemical reaction are called –
(a) Materials
(b) Products
(c) Reactants
(d) ingredients.
Answer:
(b) Products
Question (iv)
Rusting of iron is a
(a) Slow reaction
(b) Fast reaction
(c) Both (a) and (b)
(d) None of these.
Answer:
(a) Slow reaction
Question (v)
Qutub minar was built more than years ago –
(a) 1600
(b) 1650
(c) 1700
(d) 1800.
Answer:
(a) 1600
Question 2.
Fill in the blanks:
- A physical change is generally ……………..
- A chemical change is also called a …………….. reaction.
- Chemical changes are very important in our ……………..
- Ozone layer protects us from the harmful …………….. radiation which come from the sun.
- Oxygen is …………….. from ozone.
- If the content of moisture in air is high, which means if it is more humid, rusting becomes ……………..
- The process of depositing a layer of zinc on iron is called ……………..
- Stainless steel does …………….. rust.
- In chemical changes …………….. substances are produced.
- Burning of coal, wood or leaves is a …………….. change.
Answer:
- Reversible
- Chemical
- Lives
- Ultraviolet
- Different
- Faster
- Galvanisation
- Hot
- New
- Chemical.
![]()
Question 3.
Which of the following statements are true (T) or false (F):
- Conversion of milk into curd is a physical change.
- A physical change is generally reversible.
- On dissolving the ash in water it forms a new substance.
- When carbon dioxide is passed throuth lime water, calcium carbonate is formed, which makes lime water milky.
- Chemical changes are not important in our lives.
- All new substances are formed as a result of chemical changes.
- A medicine is the end product of a chain of chemical reactions.
- Stainless steel is made by mixing iron with carbon and metals.
Answer:
- False (F)
- True (T)
- True (T)
- True (T)
- False (F)
- True (T)
- True (T)
- True (T).
Question 4.
Match the items in Column A with Column B:

Answer:
(i) (b)
(ii) (d)
(iii) (a)
(iv) (c)
Physical and Chemical Changes Very Short Answer Type Question
Question 1.
Define a physical change.
Answer:
The change in which the identity of the substance does not change is called a physical change.
Question 2.
Give any one characteristic of a physical change.
Answer:
No new substance is formed as a result of physical change.
Question 3.
List two physical changes.
Answer:
- Change of an iron bar to a magnet.
- Melting of ice.
![]()
Question 4.
Complete the following:
- In a physical change the state of the substance is ………….. if cause of the change is removed.
- In a physical change only ……………… physical properties of the substance are
Answer:
- Restored
- Changed.
Question 5.
Define a chemical change.
Answer:
It is the change in which identity of the substance is changed and a new substance is formed-
Question 6.
Which of the following is true in case of a chemical change:
- Identity of the substance does not change.
- No, new substance is formed.
- Only physical properties of the substance are changed.
- Chemical properties of the substance are changed.
Answer:
4. Chemical properties of the substance are changed.
Question 7.
Give any one important characteristic of a chemical change?
Answer:
In this change new substance is formed.
Question 8.
Give any two examples of a physical change?
Answer:
- Dissolution of common salt (or sugar) in water.
- Magnetisation of an iron piece and demagnetisation of a magnet.
Question 9.
Is glowing of an electric bulb is a physical change or chemical ?
Answer:
Physical change.
![]()
Question 10.
List any two chemical changes with which we come across in our daily life.
Answer:
- Durdling of milk, and
- Digestion of food.
Question 11.
Classify the following into physical change and chemical change:
(a) Souring of kneaded flour
(b) Change of sugarcane juice to vinegar
(c) Evaporation of water
(d) Change of an iron rod to a magnet
(e) Pickling
(f) Dissolving common salt in water
(g) Change of cattle dung to biogas
(h) Glowing of an electric bulb.
Answer:
Physical change: (c), (d), (f), (h).
Chemical change: (a), (b), (e), (g).
Question 12.
Give one difference between physical and chemical change.
Answer:
In a physical change new substance is not formed, whereas in a chemical change new substance is formed.
Question 13.
Are the following changes physical or chemical:
- Burning of candle
- Preparation of soap from oil and caustic soda
- Dissolving sugar in cone, sulphuric acid, and
- conversion of grape juice to wine.
Answer:
Chemical change.
Question 14.
Pick up chemical changes out of the following:
(a) Souring of milk
(b) Making of ice – cream
(c) Burning of a candle
(d) Lighting of an electric bulb
(e) Respiration
(f) Heating of ammonium chloride
(g) Melting of wax.
(h) Breaking a chalk.
Answer:
Physical change: (b), (d), (f), (g), (h).
Chemical change: (a), (c), (e).
Question 15.
What are main points under which physical changes can be classified?
Answer:
The three main points viz. change in state, dispersion in solution, magnetisation and electrical changes.
Question 16.
Define rate of a chemical reaction.
Answer:
The rate of reaction is the quantity of products obtained per unit time from the reactant.
Question 17.
List a fast reaction.
Answer:
2Na + 2H2O → 2NaOH + H2 ↑
![]()
Question 18.
List the factors which influence the rate of a chemical reaction.
Answer:
- Nature and state of reactants
- Temperature
- Concentration of the reactants
- Catalyst.
Question 19.
What happens when temperature of a chemical reaction increases?
Answer:
Rate of reaction increases.
Physical and Chemical Changes Short Answer Type Question
Question 1.
How can you say that burning of coal and passing of electric current through water are chemical changes?
Answer:
Burning of coal:
When coal is burnt carbon dioxide gas is formed and ash is left behind as residue. Thus, new substances with new properties are formed. It is because the molecular structure of carbon-dioxide and coal are quite different from that of coal. Further more coal cannot be obtained back, hence it is a permanent change. So, it is a chemical change.
Passing of electric currnet through water:
When electric current is passed throuth water it decomposes to hydrogen and oxygen. The molecular structure of the later viz. H2 and O2 is different from that of H2O, therefore the change is a chemical one.
![]()
Question 2.
Define a physical change?
Answer:
Physical change:
A physical change is a change in the physical properties (colour, state, density etc.) and does not involve a change in molecular structure. No new substance is formed and the change can be reversed by ordinary physical means.
Question 3.
Prove that evaporation of water and heating of iron to redness are physical changes?
Answer:
When water is heated, it changes into steam. No new substance has been formed because the molecular structures of water and steam are the same. Water has merely undergone a change in form (liquid → gas).
Further steam can be converted back into water just by the process of cooling therefore, this is a temporary change. Similary when iron is heated to redness, it is a temporary change in colour because on cooling iron is obtained back in its original form.
Question 4.
How can you argue that the following changes are physical changes:
- Dissolution of sugar in water
- Melting of wax
- Lighting of bulb
- Breaking of a chalk stick.
Answer:
1. Dissolution of sugar in water:
Is a physical change because no new substance is formed. On boiling off water, sugar can be obtained back.
2. Melting of wax:
When wax is heated in a dish, it melts but its composition does not change. On cooling we get back wax.
3. Lighting of bulb:
When an electric bulb is lighted by-passing electric current, the filament glows and emits light. But no new substance is formed. So it is a temporary change.
4. Breaking of a chalk stick:
When a chalk breaks, no new substance is formed. The identity of chalk aslo does not change. Therefore, it is a physical change.
Question 5.
Dispersion of a substance is a physical change or chemical one.
Answer:
Dispersion in solution:
This is also a physical change in many cases. The solute molecules get dispersed amongst the solvent molecules. They do not suffer a change in molecular structure and the solute can be recovered unchanged by cation of the solvent.
![]()
Question 6.
Give some chemical reactions which occurs in our daily life.
Answer:
Some important chemical reactions that occur in our daily life are:
- Respiration (during respiration oxidation of glucose takes place).
- Digestion of food.
- Combustion of fuels.
- Ripening of fruits.
- Cooking of food.
- Rusting of iron.
Question 7.
Explain that change in state is nothing but a physical change?
Answer:
Change in state:
All the substances can be transformed to gases, liquids or solids by changing temperature or the other physical conditions. In the solid state the constituent particles are ordred. In the liquid state they are less ordered; in the gaseous state they are most disordered. So, the physical state of a substance can be changed by application of energy.
Thus, changes of a solid to liquid i.e., melting of a solid, change of vapour to liquid etc. can be reversed by application of enerty i.e., either by supplying heat or by cooling. This leads to the conclusion that change of state is nothing but a physical change.
Question 8.
Why heating of a metal say platinum, to redness is a physical change?
Answer:
When platinum is heated to redness it glows. This is only due to temporary excitement of electrons in its atoms. When we stop heating in electrons get unexcited and consequently it ceases glowing. Therefore, change under consideration is definitely a physical change.
Question 9.
Explain as to why the following changes are chemical changes?
- Rusting of iron
- Souring of milk
- Burning of magnesium
- Pickling.
Answer:
1. Rusting of iron:
During the phenomenon new substances viz. iron oxide etc. are formed on the surface of iron. Iron cannot be obtained back from rust. So, rusting of iron is a chemical change.
2. Souring of milk:
As a result of souring of milk new sub¬stances are formed which cannot be converted back to milk, hence souring of milk is a chemical change.
3. Burning of magnesium:
When Mg burns new substance viz. magnesium oxide is formed, which cannot be converted back to magnesium and oxygen. Therefore burning of magnesium is a chemical change.
2Mg + O2 → 2MgO
4. Pickling:
Pickling is nothing but the fermentation of the vegetable. Pickled vegetables cannot be changed back to fresh vegetables. Hence, it is also a chemical changed.
![]()
Question 10.
Give brief distinction between physical change and chemical change.
Answer:
Distinction between physical and chemical changes:
Physical Change:
- Change in physical properties.
- Temporary change.
- Composition of matter does not change.
- No new substance is formed.
- Can be reversed by physical means.
- Not accompanied by large energy changes.
- No change in weight.
Chemical Change:
- Change in chemical properties.
- More or less a permanent change.
- Composition of matter changes.
- New substance is formed.
- Cannot be reversed easily.
- Accompanied by large energy changes.
- Though total mass remains constant, mass of individual substance changes.
Question 11.
Is the solution of hydrogen chloride in water a physical changes?
Answer:
Hydrogen chloride is a covalent molecule. In solution it becomes electrovalent. The hydrogen ion combines with a water molecule to form hydronium ion. The properties of the solution are entirely different. There is a change in molecular structure and so as per our definition it is a chemical change.
Question 12.
Is an allotropic change a chemical change?
Answer:
The constituent atoms are identical in allotropes but allotropy is caused by change in molecular structure. Oxygen gas contains two atoms in each molecule but ozone contains three. In many cases the chemical as well as physical properties are different. So we have to conclude that allotropic change is a chemical change.
Question 13.
Is the cooking of rice a physical change?
Answer:
In cooking the fast molecules of boiling water pierce the walls of the cell in which strach is enclosed and release the starch. Sight hydrolysis also takes place. But the change is physical to a major extent since most of the starch remains unchanged.
![]()
Question 14.
What type of change is sublimation of ammonium chloride?
Answer:
Ammonium chloride on heating splits up into gases ammonia and hydrogen chloride. These gaseous products recombine on cooling to give the original salt. The apparent physical change is the result of two distinct chemical changes decomposition and combination. Sublimation can also be a physical change when the sub – stance is below its triple point and the change to vapour and back to solid state takes place directly, but in the case of ammonium chloride and many other compounds dissociation occurs.
Question 15.
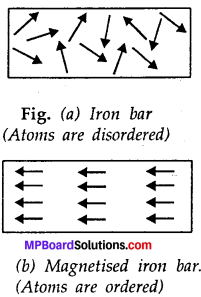
Magnetisation of an iron bar is a physical change. Explain.
Answer:
Magnetisation of an iron bar:
Magnetisation caused by alignment of constituent atoms [figs, (a) and (b)] and as such it is to be considered only as a physical change. Charging and discharging of a condenser a temporary and physical change.
Physical and Chemical Changes Long Answer Type Question
Question 1.
Give in detail the distinction between physical and chemical changes?
Answer:
Distinction between physical and chemical changes:
As already stated a chemical change involves a change in the structure of the molecules while a physical change does not involve a change in molecular structure. This definition is comprehensive and all other characteristic of a chemical change can be deduced easily as floows.
1. During a chemical change new substances with new properties are produced:
Change in molecular structure means a new substance with new properties. On the other hand, a physical change does not involve a change in the molecular structure.
2. A chemical change is a permanent change and the original substance is not got back by reversing the conditions. A physical change is temporary and exist only as long as the imposed conditions exist. A physical change can be reversed by reversing the conditions.
3. No change, physical or chemical can take place without energy change. A large amount of heat energy has to be given to convert water into steam. But the energy involved in chemical changes is generally far greater than that involved in physical changes.
4. A chemical change means a physical change also, because won news substances with new properties are produced the physical state and physical properties are altered.
![]()
Question 2.
Give a detailed account of modem approach of physical and chemical changes?
Answer:
Modern approach of physical and chemical changes:
As your studies in science progress you will appreciate the point of view that all changes are chemical changes. Chemical changes are changes in structure. Minor electronic changes do occure even when a physical change takes place. In solids, subjected to heat, the ions oscillate with increasing amplitude and electrons move with greater speeds in expanded orbitals. Breaking a piece of diamond is an accepted chemical change because covalent chemical bonds are broken in the process. The same is happening when any substance is broken.
Thedivision into physical and chemical changes is, therefore, purely a matter of convenience. The three types of changes are classified as fallows:
1. Physical change:
Slight changes in outer electron states. Comparatively less energy change. No change in molecular patterns. No change in nucleonic pattern.
2. Chemical change:
Change in molecular pattern. Large scale energy change. Rearrangement, of electronic orbitals. No change in nucleonic pattern.
Question 3.
What are slow and fast reactions? Give example.
Answer:
A reaction can be called slow or fast depending upon the speed rate of reaction.
Whenever elements and compounds react their reaction is said to be slow if the rate of the reaction, i.e., the quantity of products obtained per unit time from the reactants, is slow.
Example:
- Rusting of iron is a slow reaction.
- When a copper vessel kept in moist air it gets greenished due to the formation of basic copper carbonate. To the contrary if the speed or the rate of the reaction is fast, than the reaction is said to be the fast reaction.
Example:
By adding sodium chloride in silver nitrate solution, immediately a white ppt. appears. It is a fast reaction i.e.,
![]()
Question 4.
What is a chemical reaction? Give an example.
Answer:
A reaction in which the following are being occurred is called a chemical reaction:
- Absorption or liberation of heat or any other form of energy.
- Change in the identity of the combining substances i.e., the reactants.
- Collision among the reactant molecules.
- Use of some catalyst or provision of some form of energy to the reactants for initiation of reaction.
Example:
1. Sodium reacts with water to form sodium hydroxide alongwith the liberation of hydrogen gas.
The chemical equation of the reaction is as follows:
2Na + 2H2O → NaOH + H2
2. Lime stone, on heating, liberates carbon dioxide.
The equation for the reaction is as follows:
CaCO3 → CaO + CO2.