MP Board Class 12th Chemistry Solutions Chapter 3 Electrochemistry
Electrochemistry NCERT Intext Exercises
Question 1.
How would you determine the standard electrode potential of the system Mg2+ | Mg ?
Answer:
\(\mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}^{\circ}\) can be determined by forming a cell with standard hydrogen electrode (SHE).
Mg(s) | Mg2+ 1 M || H+g | H2 (1 atm) Pt.
Question 2.
Can you store copper sulphate solutions in a zinc pot ?
Answer:
No, it is not possible. The E° values of the copper and zinc electrodes are as follows :
Zn2+(aq) + 2e– → Zn(s) ; E° = – 0·76 V
Cu2+(aq) + 2e– → Cu(s) ; E° = + 0·34 V
This shows that zinc is a stronger reducing agent than copper. It will lose electrons to Cu2+ ions and redox reaction will immediately set in.
Zn(s) + Cu2+ (aq) → Zn2+(aq) + Cu(s)
Thus, copper sulphate solution cannot be stored in zinc pot.
Question 3.
Consult the table of standard electrode potentials and suggest three sub-stances that can oxidise ferrous ions under suitable conditions.
Answer:
Only those species can oxidise ferrous ions (Fe2+) whose standard reduction potential is more positive than 0.77V. Thus, suitable oxidising agents will be F2, Cl2, Br2.
Question 4.
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Solution:
For hydrogen electrode, H+ + e– → 1/2H2
Applying Nernst equation,
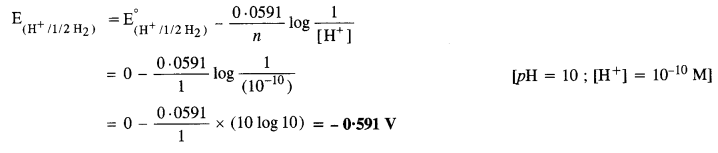
Question 5.
Calculate the emf of the cell in which the following reaction takes place :
Ni(s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag(s) Given that E∘cell = 1.05 V.
Solution:
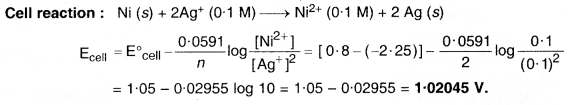
Question 6.
The cell in which the following reactions occurs :
2Fe3+(aq) + 2I–(aq) → 2Fe2+(aq) + I2(s) has E∘cell = 0-236 V at 298 K.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Solution:
The two half reactions are :
2Fe3+ + 2e– → 2Fe2+ and 2I– → I2 + 2e–
For the above reaction, n = 2
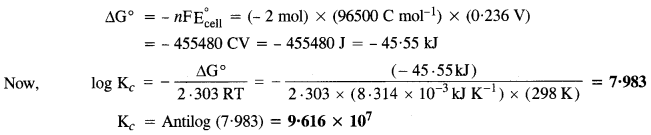
Question 7.
Why does the conductivity of a solution decreases with dilution ?
Answer:
Because number of ions per cm3 decreases.
Question 8.
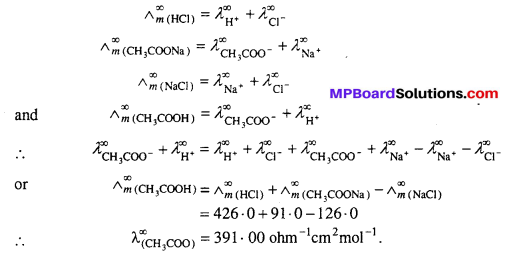
Suggest a way to determine the \(\wedge_{m}^{0}\) value of water.
Answer:
Conductance of weak electrolytes can be determined by kohlraush’s law. Thus, molar conductance of water of infinite dilution can be determined by the molar conductances of NaOH, HCl and NaCl at infinite dilution.
![]()
Question 9.
The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1 Calculate its degree of dissociation and dissociation constant Given \(\lambda_{\left(\mathbf{H}^{+}\right)}^{\circ}\) = 349.6 S cm2 mol-1 and \(\lambda_{\left(\mathrm{H} \mathrm{COO}^{-}\right)}^{\circ}\) = 54.6 S cm2 mol.
Solution:
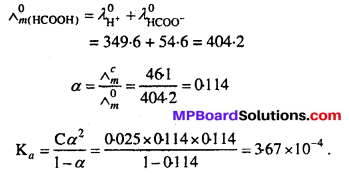
Question 10.
If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire ?
Solution:
Q (coulomb) = 1 (ampere) × t (sec)
Q = 0.5 ampere × 2 × 60 × 60
= 3600 C
A flow of IF, i.e. 96500 C is equivalent to flow of 1 mole of electrons
i. e., = 6.023 × 1023 electrons
3600 C is equivalent to flow of electrons
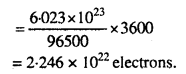
Question 11.
Suggest a list of metals that are extracted electrolytically.
Answer:
The metals like Na, K, Mg, Al, Ca etc. i.e., reactive metals can be extracted electrolytically.
Question 12.
What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2O-27 ? Consider the reaction :
![]()
Answer:
1 mole Cr2O-27 requires 6 moles electrons for reduction.
∴ Required charge = 6F
= 6 × 96500 coulomb.
= 579000 coulomb.
Question 13.
Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging.
Answer:
Lead storage battery : It is a secondary cell i.e., a cell which is rechargeable because the products of cell reaction sticks to the electrode. It is also called as lead storage cells. It consists of six cells connected in series. Each cell consist of spongy lead anode and a grid of lead packed with lead dioxide (PbO2) acts as cathode. An aqueous solution of H2SO4 (38% by mass) acts as electrolyte. The reactions which takes place at electrodes can be represented as:

Concentration of H2SO4 decreases as sulphate ions are consumed to form PbSO4 during the working of the cell. As a result of this the density of solution also decreases.
Recharging the cell / battery: Lead storage battery can be recharged by connecting it to an external source of direct current. This reverses the flow of electron with the deposition of Pb on the anode and PbO2 on the cathode. That is, during recharging operation the cell behaves as electrolytic cell. Following reaction occurs during recharging.
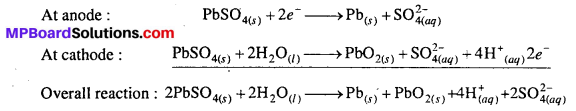
Question 14.
Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Answer:
Methane, Methanol.
Question 15.
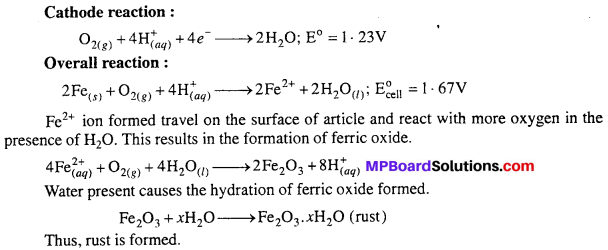
Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
Answer:
Formation of carbonic acid takes place on the surface of iron
![]()
In presence of H+ ion, oxidation of iron takes place
![]()
The electrons are used at other spot where reduction takes place.
![]()
Overall reaction,
![]()
![]()
Electrochemistry NCERT TextBook Exercises
Question 1.
Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg and Zn.
Answer:
A metal with lesser standard potential (more reactive) can displace the other metal from solution of its salts.
![]()
Question 2.
Given the standard electrode potentials, K+/K = -2.93V, Ag+/Ag = 0.80V, Hg2+/Hg = 0.79V, Mg2+/Mg = -2.37 V, Cr3+/Cr = -0.74V. Arrange these metals in their increasing order of reducing power.
Answer:
Less the electrode potential, more will be the reducing power.
Ag < Hg < Cr < Mg < K.
Question 3.
Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq)+ 2Ag(s) takes place. Further show :
(i) Which of the electrode is negatively char-ged ?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Answer:
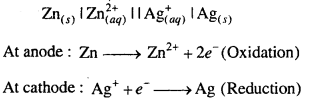
(i) Anode i.e., Zn electrode is negatively charged.
(ii) Electrons are current carriers.
Question 4.
Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
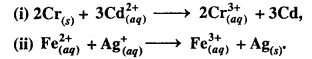
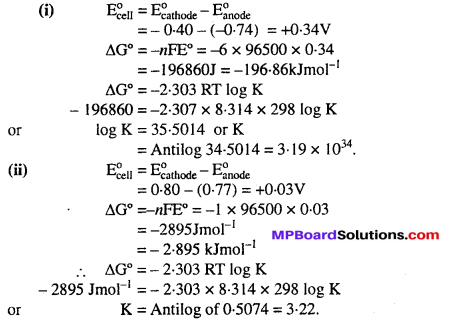
Calculate the ∆rG°, and equilibrium constant of the reactions.
Solution:
Question 5.
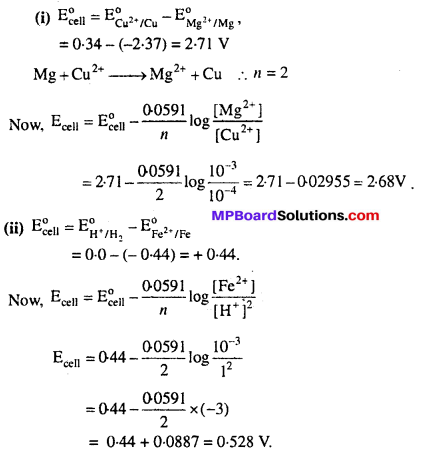
Write the Nernst equation and emf of the following cells at 298 K :
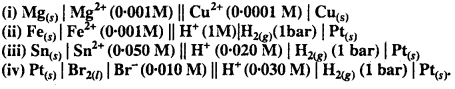
Solution:
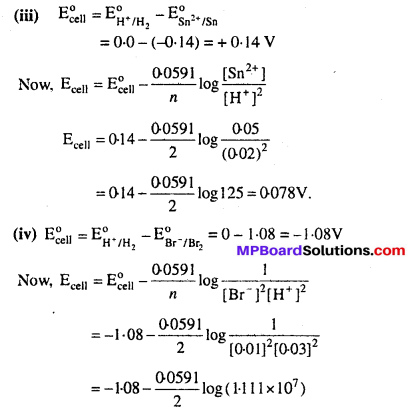
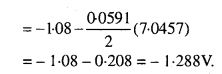
Question 6.
In the button cells widely used in watches and other devices the following reaction takes place:
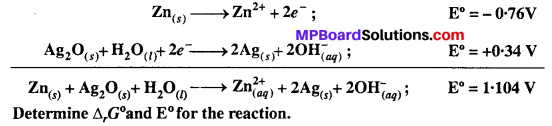
Solution:

Question 7.
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Answer:
Conductivity : Conductivity of a solution is defined as the conductance of a solution of 1 cm length and having 1 sq. cm as the area of cross-section.
Molar conductivity : Molar conductivity of a solution at a dilution (V) is the conductance of all the ions produced from one mole of the electrolyte dissolved in V cm3 of the solution when the electrodes are one cm apart and area of cross-section of the electrodes is so large that the whole of the solution is contained between them. It is usually represented by Λm.
Variation with concentration : The conductivity of a solution (Both for strong and weak electrolytes) decreases with decrease in concentration of the electrolyte i.e., on dilution. This is due to the decrease in the number of ions per unit volume of the solution on dilution. The molar conductivity of a solution increase in the decrease in concentration of the electrolyte i.e., on dilution. This is due to the decrease in the number of ions per unit volume of the solution on dilution. The molar conductivity of a solution increases with decrease in concentration of the electrolyte. This is because both number of ions as well as mobility of ions increases with dilution. When concentration approaches zero, the molar conductivity is known as limiting molar conductivity.
Question 8.
The conductivity of 0.20 M solution of KCI at 298 K is 0.0248 Scm-1. Calculate its molar conductivity.
Solution:
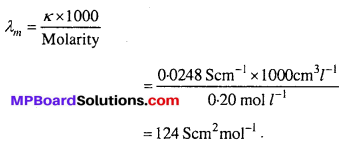
Question 9.
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500Ω What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1.
Solution:
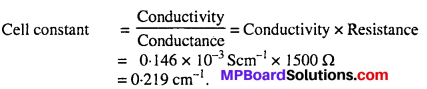
Question 10.
The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below :
Concentration/M 0.001 0.010 0.020 0.050 0.100 102 × kSm-1 1.237 11.85 23.15 55.53 106.74 for all concentrations and draw a plot between Λm and C1/2 Find the value calculate Λm and C1/2 Find the value calculate Λm of Λ0m.
Answer:
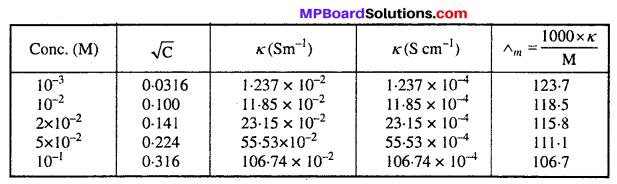
Λ0m = intercept on Λm axis = 124-0 S cm2 mol-1 (on extrapolation to zero concentration)
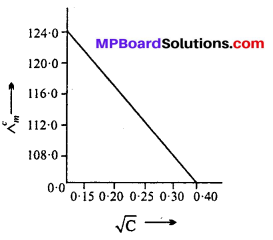
![]()
Question 11.
Conductivity of 0.00241 M acetic acid is 7.896 × 10-5 S cm-1. Calculate its molar conductivity and if Λ0m for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant ?
Solution:
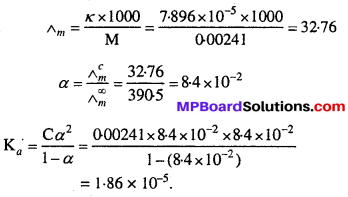
Question 12.
How much charge is required for the following reductions :
(i) 1 mol of Al3+ to Al.
(ii) 1 mol of Cu2+ to Cu.
(iii) 1 mol of MnO–4 to Mn2+.
Solution:
(i) The electrode reaction is :

(ii) The electrode reaction is :
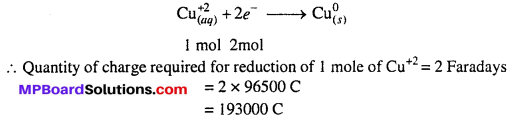
(iii) The electrode reaction is :

Question 13.
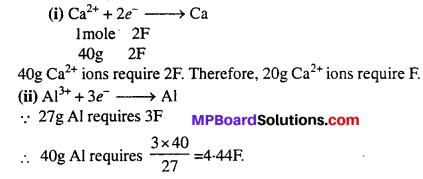
How much electricity in terms of Faraday is required to produce (i) 20.0 g of Ca from molten CaCl2.
(ii) 40.0 g of Al from molten Al2O3.
Solution:
Question 14.
How much electricity is required in coulomb for the oxidation of (i) 1 mole of H2O to O2
(ii) 1 mole of FeO to Fe2O3.
Answer:
(i) 2H2O → O2 + 4H+ + 4e–
2mole – 4F
lmole – 2F
Electricity required for one mole H2O = 2F = 2 × 96500 coulomb.
(ii) 4FeO + O2 → 2Fe2O3
or Fe2+ → Fe3+ + e–
1 mole – IF
1 × 96500 coulomb.
Question 15.
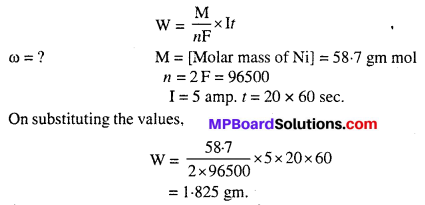
A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode ?
Answer:
According to Faraday’s first law:
W = ZIr [z = \(\frac { M }{ nF } \)]
Question 16.
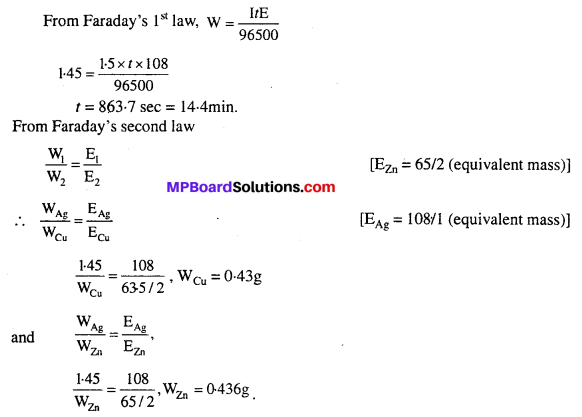
Three electrolytic cells A, B, C containing solutions of ZnSO4, AgNO3 and CuSO4, respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow ? What mass of copper and zinc were deposited ?
Solution:
Question 17.
Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible :
(i) Fe3+(aq) and I–(aq)
(ii) Ag+(aq) and Cu(s)
(iii) Fe3+(aq) and Br–(aq)
(iv) Ag(s) and Fe3+(aq)
(v) Br2(aq) and Fe2+(aq)
Answer:
A reaction is feasible if EMF of the cell is +ve.
Cathode : At which reduction occurs.
Anode : At which oxidation occurs.
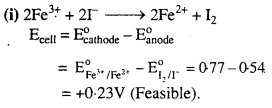
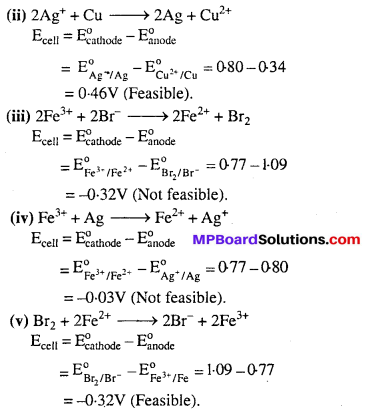
![]()
Question 18.
Predict the products of electrolysis in each of the following :
(a) An aqueous solution of AgNO3 with silver electrodes.
(b) An aqueous solution of AgNO3 with platinum electrodes.
(c) A dilute solution of H2SO4 with platinum electrodes.
(d) An aqueous solution of CuCl2 with platinum electrodes.
Answer:
(a) At cathode : Possible reactions are :
(i) Reduction of Ag+ E°Ag = 0.80V
(ii) Reduction of H2O E°H2O = -0.83 V
∴ Ag+ ion reduced because its E°Ag > E°H2O
At anode : Possible reactions are :
(i) Oxidation of H2O E° = 1.23 V
(ii) Oxidation of Ag E° = 0.80V
Since E°Ag < E°H2O ∴ Ag gets oxidised.
(b) Possible reactions at cathode :
(i) Reduction of Ag+ E°g = 0.80V
(ii) Reduction of water E°H2O = -0.8 V .
Since E°Ag > E°H2O ∴Ag+ gets reduced.
Possible reactions at anode :
Oxidation of water E°Ag = 1.23
∴ O2 gas is liberated at anode.
(c) Possible reaction at cathode :
(i) Reduction of H+ E°H+/H2 0.0
(ii) Reduction of H2O E°H2O = -0.87
Since E°H+/H2 > E°H2O ∴ H2 gas is liberated at cathode.
Possible reaction at anode :
Oxidation of water E°H2O = 1.23
∴ O2 gas is liberated at anode.
(d) Possible reaction at cathode :
(i) Reduction of water E° H2O = -0.87V
(ii) Reduction of Cu2+ E°Cu2+/Cu = 0.34V
Since E°cu2+/cu > E°H2O ∴ Cu2+ gets reduced at cathode.
Possible reaction at anode :
(i) Oxidation of water E°H2O = 1.23V
(ii) Oxidation of Cl- E° Cl–/Cl2 = 1.36V
Cl2 gas is liberated at anode because oxidation of water is kinetically slow.
![]()
Electrochemistry Other Important Questions and Answers
Electrochemistry Objective Type Questions
Question 1.
Choose the correct answer:
Question 1.
If specific conductance = K, R = Resistance, l = distance between the electrodes A = Cross-sectional area of a conductor Cm = mol L-1, Ceq = g.eq L-1 then specific resistance of electrolyte will be equal to :

Question 2.
K is equal to :

Question 3.
Molar conductivity Λm is equal to :
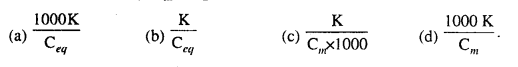
Question 4.
Cell constant is :
![]()
Question 5.
For electrolyte (v+ = v– = 1) like NaCI:
![]()
Question 6.
Which among the following is not a conductor of electricity :
(a) NaCl(aq)
(b) NaCl(s)
(c) NaCl(mol)
(d) Ag.
Question 7.
By which of the following the resistance of the solution is multiplied to obtain cell constant:
(a) Specific conductance (K–)
(b) Molar conductance (Λm)
(c) Equivalent conductance (Λeq)
(d) None of these.
Question 8.
Increase in equivalent conductance of the solution of an electrolyte by dilution is due to:
(a) Increase in ionic attraction
(b) Increase in molecular attraction
(c) Increase in association of electrolyte
(d) Increase in ionization of electrolyte.
Question 9.
If the specific conductance and observed conductance of an electrolyte is same then its cell constant will be :
(a) 1
(b) 0
(c) 10
(d) 1000
Question 10.
Unit of cell constant is :
(a) ohm-1 cm-1
(b) cm
(c) ohm cm
(d) cm-1.
Question 11.
Unit of specific conductance is :
(a) ohm-1
(b) ohm-1 cm-1
(c) ohm-2 cm-1 equivalent-1
(d) ohm-1 cm-2.
Question 12.
If the concentration of any solution is C gram equivalent/ litre and specific resistance is A, its equivalent conductance will be :
![]()
Question 13.
The coating of layer of zinc on iron to prevent it from corrosion is called :
(a) Galvanization
(b) Cathodic protection
(c) Electrolysis
(d) Photoelectrolysis.
Question 14.
A saturated solution of KNO3 is used for salt bridge because :
(a) Speed of K+ is more than NO–3
(b) Speed of NO–3 is more than K+
(c) Speed of both is nearly same
(d) Solubility of KNO3 is high in water.
Question 15.
Acts as dipolar in dry cells :
(a) NH4Cl
(b) Na2CO3
(c) pbSO4
(d) MnO2
Question 16.
Reduction is known as :
(a) Electronation
(b) De-electronation
(c) Protonation
(d) De-protonation.
Question 17.
What is the path of electric current in a Daniel cell when Zn and Cu electrodes are connected:
(a) From Cu to Zn inside the cell
(b) From Cu to Zn outside the cell
(c) From Zn to Cu inside the cell
(d) From Zn to Cu outside the cell.
Question 18.
In a cell containing Zn electrode and normal hydrogen electrode (NHE), Zn acts like:
(a) Anode
(b) Cathode
(c) Neither cathode nor anode
(d) Both anode and cathode.
Question 19.
If salt bridge is removed from half cells then voltage :
(a) Reduces and becomes zero
(b) Increases
(c) Immediately increases
(d) Does not change.
Question 20.
When lead storage battery is discharged :
(a) SO2 is released
(b) Pb is manufactured
(c) PbSO4 is used
(d) H2SO4 is used.
Question 21.
Process of Rusting of iron is :
(a) Oxidation
(b) Reduction
(c) Corrosion
(d) Polymerisation.
Question 22.
Value of standard potential of hydrogen electrode is :
(a) Positive
(b) Negative
(c) Zero
(d) No definite value.
Answers:
1. (b), 2. (c), 3. (d), 4. (b), 5. (a), 6 (b), 7. (a), 8. (d), 9. (a), 10. (d), 11. (b), 12. (a), 13. (a), 14. (c), 15. (d), 16. (a), 17. (d), 18. (a), 19. (a), 20. (d), 21. (c), 22. (c).
Question 2.
Fill in the blanks :
- Acetic acid is a ………………… electrolyte.
- Conductance of electrolyte ………………… with the increase in temperature.
- On increasing dilution the value of specific conductance of a solution …………………
- ………………… decreases with increase in size of ion.
- Unit of specific resistance is …………………
- Primary cells cannot be ………………… again.
- Device which converts chemical energy into electrical energy is known as ………………… cell.
- Amount of electric current which produces one gram equivalent of a substance is known as …………………
- In metallic conduction ………………… property remains unchanged.
- Reciprocal of resistance is known as …………………
- Conductance of 1 cm cube of a conductor is called …………………
- 1 Faraday is equal to ………………… coulomb.
- Rusting of iron is an example of …………………
- Potential of standard hydrogen is assumed to be …………………
Answers:
- Weak
- Increases
- Decreases
- Conductance
- ohm cm
- Charged
- Electrochemical
- Faraday
- Chemical property
- Conductance
- Specific conductance
- 96500 coulomb
- Corrosion process
- 0.0 volt
- Electrochemical cell.
Question 3.
Match the following :
I.
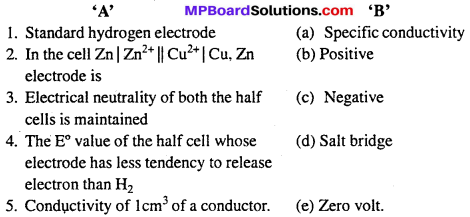
Answers:
- (e)
- (c)
- (d)
- (b)
- (a).
II.


Answer:
- (c)
- (d)
- (b)
- (a)
- (e)
- (f).
Question 4.
Answer in one word / sentence :
1. Give two examples of strong electrolyte.
2. Give two examples of weak electrolytes.
3. What is the effect of temperature on electrolytic conductivity ?
4. Write the formula of Kohlrausch’s law.
5. Write the formula of equivalent conductance.
6. Write the formula of molar conductance.
7. Device which converts electrical energy into chemical energy.
8. What is the potential of both the electrodes of the cell due to which electric current flows in the cell ?
9. What is the potential produced due to redox reaction between a metal electrode and its ions called ?
10. What is the unit of potential difference ?
11. Write the formula of cell constant.
12. Cell which can be recharged are known as.
13. State the unit of Equivalent conductance.
14. What is the chemical composition of rust ?
15. Write the relation between Electromotive force and Equilibrium constant of a cell.
16. What is the name of the reaction in which oxidation and reduction occur simultaneously ?
Answers:
1. Strong electrolyte : HCl, NaOH, NaCl
2. Weak electrolyte : CH3COOH, H2CO3,
3. Conductivity increases
4.
![]()
5. Equivalent conductance
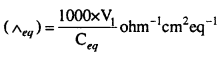
6.
![]()
7. Electrolytic cell
8. Electromotive force
9. Electrode potential
10. Volt
11. Cell constant = \(\frac { l }{ A } \)
12. Secondary cell
13. Ohm cm2 gm eq-1
14. Fe2O3 . xH2O
15.
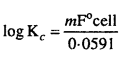
16. Redox reaction.
![]()
Electrochemistry Very Short Answer Type Questions
Question 1.
Write the definition of Electrochemical cell.
Answer:
System in which chemical energy is converted to electrical energy by oxidation reduction is known as electrochemical cell or voltaic cell.
Question 2.
What is an Electrolytic cell ?
Ans.
The container or system in which electrical energy is passed by which chemical reaction takes place thus, electrical energy is converted to chemical energy is known as electrolytic cell.
Question 3.
What is Electrode potential ?
Answer:
The potential difference developed between the electrodes and electrolyte of an Electrolytic cell is known as Electrode potential.
Question 4.
What is a strong electrolyte ? Write two examples.
Answer:
Electrolyte which completely dissociate in aqueous solution are known as strong electrolyte.
Example : NaCl, KCl, NH4Cl etc.
Question 5.
What is a weak Electrolyte ? Write two examples.
Answer:
Electrolyte which dissociate partially in aqueous solution are known as weak electrolyte.
Example : NH4OH, CH3COOH, HCN etc.
Question 6.
What is meant by standard electrode potential ?
Answer:
Standard electrode potential (E°) of a half cell is the potential difference when one electrode is dipped in molar solution of its ion at 298 K. If electrode is gaseous the pressure of gas must be one atmosphere.
In IUPAC system, reduction potential are known as standard electrode potential.
Question 7.
Write Ohm’s law.
Answer:
According to Ohm’s law “It states that potential difference across the conductor is directly proportional to the current (I) flowing through it” i.e.,
Mathematically, it can be written as :
I α V
V = IR (R = Resistance, unit = ohm, Ω)
Question 8.
What is cell constant ?
Answer:
For a conductivity cell, the ratio of distance between two electrodes (l) and area of cross-section of electrode (A) is called as cell constant.
∴ Cell constant = \(\frac { l }{ A } \) or x = \(\frac { l }{ A } \)
Unit of cell constant = cm-1.
Question 9.
What is galvanization ? Explain.
Answer:
Iron is coated with the layer of zinc to protect it from rusting. This process is known as galvanization. The galvanized iron articles keep their lustre due to the coating of invisible protective layer of basic zinc carbonate, ZnCO3.Zn(OH)2.
Question 10.
What is Electrochemical Equivalent ?
Answer:
Electrochemical equivalent of a substance is that mass of substance released or deposited on an electrode when a current of one ampere is passed for one second.
![]()
Electrochemistry Short Answer Type Questions
Question 1.
What is salt bridge ? Write its two functions.
Answer:
‘U’ shaped tube filled with KCl or KNO3 in Agar-Agar solution or gelatin, is known as salt bridge. It connects the two half cell.
Functions : (i) It allows the flow of current by completing the circuit.
(ii) It maintains the electrical neutrality.
Question 2.
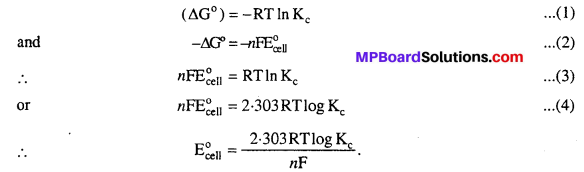
Derive relation between standard electromotive force and equilibrium constant
Answer:
Relation between standard electromotive force and equilibrium constant can be derived using van’t Hoff isochore. For any given reaction equilibrium constant IQ is equal to the ratio of rate constant of forward reaction and rate constant of backward reaction.
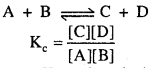
Value of equilibrium constant Kc can be calculated using standard free energy change (∆G°) because
Question 3.
What do you understand by oxidation-reduction reactions ?
Answer:
Oxidation-Reduction reactions: Chemical reactions in which valency of elements changes are known as oxidation-reduction reactions. In this process both oxidation and reduction reactions occur simultaneously, in which one of the substance is oxidized and the other substance is reduced. Like
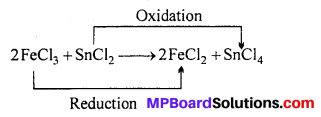
In this reaction FeCl3 is reduced to FeCl2 and SnCl2 is oxidized to SnCl4. In the reaction valency of Fe decreases and valency of Sn increases.
Question 4.
Write difference between Metallic conduction and Electrolytic conduction.
Answer:
Differences between Metallic conduction and Electrolytic conduction :
Metallic conduction:
- Metallic conduction takes place by movement of electrons.
- There is no chemical change.
- There is no transfer of matter.
- In metallic conduction conductivity decreases with increase in temperature.
Electrolytic conduction
- Electrolytic conduction takes place by movement of ions.
- Due to chemical change decomposition of electrolyte takes place.
- Transfer of matter takes place as ions.
- In electrolytic conduction conductivity increases with increase in temperature.
Question 5.
What are the difference between emf (Cell potential) and potential difference
Answer:
Difference between EMF and Potential difference :
EMF / Cell potential:
- It is the potential difference between the two terminals of the cell when no current is flowing in the circuit, i.e., in an open circuit.
- It is the maximum voltage which can be obtained from a cell.
- It can be measured by potentiaometrie method.
- Work performed by electromotive force is the maximum work done by a cell.
- It is responsible for continuous flow of current in electric circuit.
Potential difference:
- It is the difference of the electrodes potentials of the two electrodes when the cell is sending current through the circuit.
- It is the less than the maximum voltage as it is the difference of electrode potential.
- It can be measured by simple voltmeter also.
- Work performed by potential difference is less than the maximum work done by a cell.
- It is not responsible for the continuous flow of current in circuit.
Question 6.
What is specific conductance ? Give its unit.
Answer:
Specific conductivity: The reciprocal of resistivity is called specific conductiv¬ity. It is defined as the conductance between the opposite faces of one centimeter cube of a conductor. It is denoted by K (kappa).
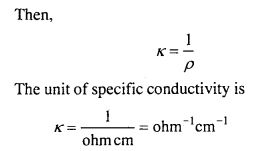
The specific conductivity of a solution at a given dilution is the conductance of one cm cube of the solution. It is represented by K (kappa).
Note : The specific conductivity of a solu¬tion of electrolyte depends upon the dilution or molar concentration of the solution.
Question 7.
What is resistivity of any solution ?
Answer:
Resistivity : When current flow in the solution through two electrodes the resistance is proportional to length and inversely proportional to cross-sectional area A.

The constant p (rho) is called resistivity or specific resistance.
Unit: If l is expressed in cm, A in cm2 and R in ohm, the unit of resistivity will be

or Resistivity of any solution is the resistance of 1 cm cube.
Question 8.
Differentiate between Electrochemical cell (Galvanic cell) and Electrolytic cell.
Answer:
Differences between Electrochemical and Electrolytic cells :
Electrochemical cell or Galvanic cell:
- It is a device to convert chemical energy into electrical energy.
- It consists of two electrodes in different compartments joined by a salt bridge.
- Redox reactions occurring in the cell are spontaneous.
- Free energy decreases with operation of cell, i.e., ∆G < 0.
- Useful work is obtained from the cell.
- Anode works as negative and cathode as positive electrodes.
- Electrons released by oxidation process at anode go into external circuit and pass to cathode.
- To set-up this cell, a salt bridge/porous pot is used.
Electrolytic cell:
- It is a device to convert electrical energy into chemical energy.
- Both the electrodes are in same solution.
- Redox reactions occurring in the cell are non-spontaneous.
- Free energy increases with operation of cell, i.e., ∆G > 0.
- Work is done on the system.
- Anode is positive and cathode is negative.
- Electrons enter into cathode electrode from external source and leave the cell at anode.
- No salt bridge is used in this cell.
Question 9.
What is equivalent conductance ?
Answer:
Equivalent conductance:
“Conductance of total ion produced by one gram equivalent of electrolyte in the solution is called equivalent conductance.” It is denoted by Λeq.
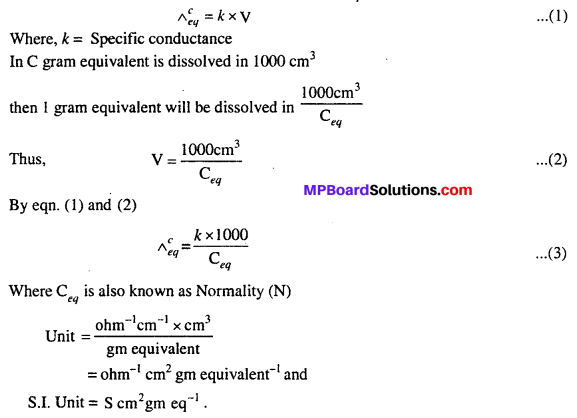
Question 10.
What is molar conductance ?
Answer:
Molar Conductivity : The molar conductivity of a solution at definite concentration (or dilution) and temperature is the conductivity of that volume which contains one mole of the solute and is placed between two parallel electrodes 1 cm apart and having sufficient area to hold whole of the solution. It is denoted by Λm.
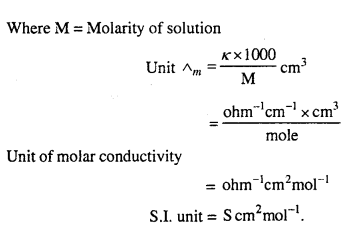
Mathematically,
Λm = K × V …(1)
Where V is the volume in ml in which one gram mole of substance is dissolved.
If M is molarity or m moles are dissolved in 1000 ml.

Question 11.
Define cell constant Develop a relation between specific conductance and cell constant.
Answer:
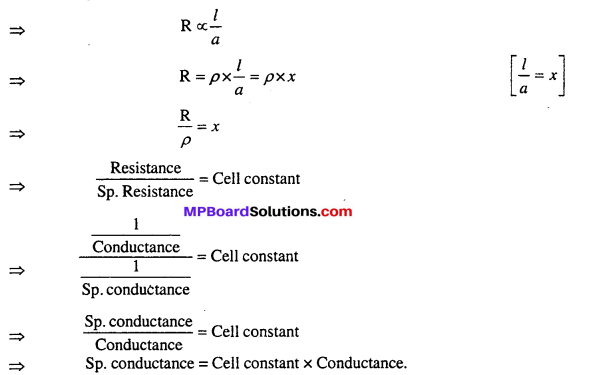
Cell constant: In any conductive cell, the distance between two electrodes and surface area of electrode A are constant. The ratio of l and a is called cell constant i.e.
![]()
Unit of cell constant is cm–1 and it is generally expressed by x.
Relation between specific conductance and cell constant : For a conductor, the resistance R is directly proportional to length R and inversely proportional to area of cross – section of electrolyte.
Question 12.
What are the factors which influence the electrical conductance of electrolytes ?
Answer:
Factor which influence electrical conductivity of electrolytes :
The main factor which influence the electrical conductivity are following:
1. Temperature: It influence following interactions.
(a) Interionic attractions: It depends upon the solute-solute interactions. Which is found between the ions of solute.
(b) Solvation of ions: It depends upon solute-solvent interactions. It is relation between ions of solute and solvent molecules.
(c) Viscosity of solvent: It depends upon solvent-solvent interactions. Solvent molecules are related with each other.
With increase in temperature all these three effects decrease and average kinetic energy of ions increases. Thus, with increase of temperature, resistance of solution decreases and hence conductance increases.
2. Nature of electrolyte : The conductance of solution depends upon the nature of electrolyte. On the basis of conductance measurement electrolytes are classified as strong electrolyte and weak electrolyte. Strong electrolytes have high value of conductance even at higher concentration also.
3. Dilution or concentration : It is main factor which influence electrical conductance. Effect of dilution or concentration can be studied indivisually in equivalent conductance, specific conductance and molar conductance. But for a general concept of electrical conductance of solution as the concentration is lowered or dilution increases, electrical conductance of whole solution increases.
Question 13.
On what factors does the various conductivities of an electrolytic solution depend ?
Answer:
Conductivities of electrolytic solution depend on the following factors :
- Dilution : On increasing dilution, value of specific conductance of a solution decreases, value of equivalent conductance and molar conductance increases.
- Nature of solvent: A solvent with high dielectric constant has high conductivity and with low dielectric constant has low conductivity.
- Number of ions present in solution: Conductivity of strong electrolytes is higher than the conductivity of weak electrolytes.
- Size of ion : In aqueous solution, small ions are heavily hydrated due to which their conductivity decreases.
- Effect of Temperature: With the increase in temperature conductivity increases.
Question 14.
With the increase in dilution how do specific conductance, Equivalent conductance and molar conductance change ?
Answer:
With the increase in dilution, specific conductance decreases. This is because by the increase in dilution number of ions present in 1 cm cube of solution decreases.
But, Equivalent conductance Λeq = K × V
and Molar conductance Λm = K × V
Equivalent conductance and molar conductance are the product of specific conductance and dilution. By the increase in dilution (or decrease in concentration) magnitude of K decreases but that of V increases.
Increase in magnitude of V is comparatively much more than the decrease in magnitude of K. Thus, by the combined effect of both, by the increase in dilution Λeq and Λm increases.
![]()
Question 15.
What is an Electrolytic cell and how does it work ?
Answer:
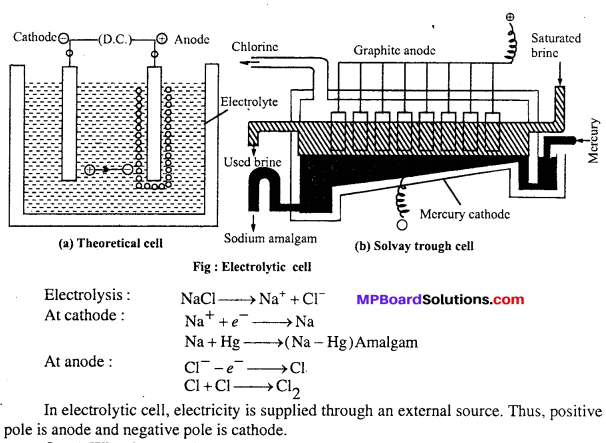
Electrolytic cells : In these cells electric current is supplied through an external source, as a result of which chemical reactions take place which is called electrolysis like : Electrolysis of water, NaCl, Al2O3 etc. For example in Solvay trough cell electrode is immersed in sodium chloride solution and electric current is passed due to which NaCl electrolyses.
At mercury cathode sodium is released and at anode chlorine is released. Sodium forms amalgam with mercury and is taken out of the cell.
Question 16.
What is meant by electromotive force of an electrochemical cell ?
Answer:
The difference in electrode potentials of the two electrodes of an electro- chemical cell is known as electromotive force or cell potential. It is expressed in volt.
Due to difference in potential electric current flows from an electrode of lower potential to an electrode of higher potential. EMF of the cell can be expressed in terms of reduction potential as :
Cell potential = Standard electrode potential – Standard electrode potential
of R.H.S. electrode of L.H.S electrode
![]()
EMF of a cell is measured by connecting the voltmeter between the two electrodes of a cell. EMF of a cell depend on the concentration of solutions of both half cells and nature of the two electrodes. For example, In Daniel cell, concentration of CuSO4 and ZnSO4 solutions in the two half cells is 1M and at 298 K EMF of the cell is 1.10 volt.
![]()
Electrochemistry Long Answer Type Questions
Question 1.
What is standard hydrogen electrode ? How is it prepared ?
Answer:
Standard hydrogen electrode : This consists of gas at 1 atmospheric pressure bubbling over a platinum electrode immersed in 1 M HC1 at 25°C (298 K) as shown in figure. The platinum electrode is coated with platinum black to increase its surface. The hydrogen electrode thus con¬structed forms a half cell which on coupling with any other half cell begins to work on the principle of oxidation or reduction. Electrode depending upon the circumstances works both as anode or cathode.
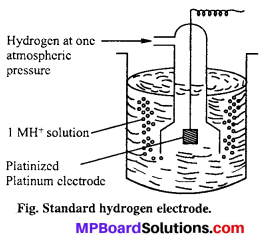
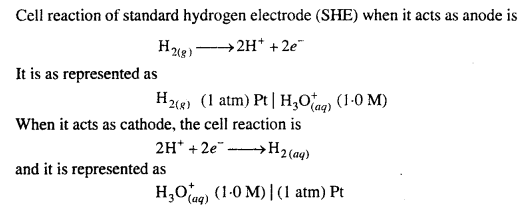
Standard hydrogen electrode (SHE) is arbitrarily assigned a potential of zero.
Question 2.
Derive Nernst Equation for single electrode potential.
Answer:
Value of standard electrode potential given in electrochemical series is applicable only when the concentration of electrolyte is 1M and temperature is 298 K. But in electrochemical cells the concentration of electrolyte is not definite and electrode potential depends on concentration and temperature. In such condition single electrode potential can be expressed by Nernst equation.
For a reduction half reaction, Nernst equation can be expressed as follows :
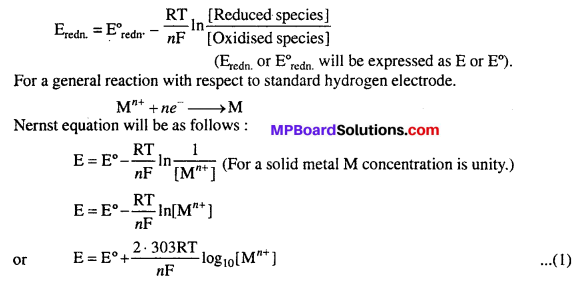
Where E = Reduction electrode potential
E° = Standard electrode potential (Mn+ concentration 1M and at 298 K)
R = Gas constant = 8.31 JK-1 mol-1 = Temperature (in kelvin) = 298 K
n = Valency of metal ion, F = 1 Faraday (96,500 coulomb)
![]()
Equation (2) is Nernst equation for single electrode potential.
Question 3.
Write the Faraday’s laws of electrolysis.
Answer:
Faraday’s first law of electrolysis : The law states that, “The mass of any substance deposited or liberated at any electrode is directly proportional to the quantity of electricity passed.”
Thus, if W gm of the substance is deposited on passing Q coulomb of electricity, then
W α Q or W = ZQ
Where, Z is a constant of proportionality and is called electrochemical equivalent of the substance deposited. If a current of I ampere is passed for t second, then Q = I × t. So that,
W = Z × Q = Z × I × t
Thus, if Q = 1 coulomb, I = 1 ampere and t = 1 second, then W = Z. Hence, electrochemical equivalent of a substance may be defined as, “The mass of the substance deposited when a current of one ampere is passed for one second.”
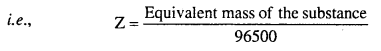
As one faraday (96500 C) deposits one gram equivalent of the substance, hence electrochemical equivalent can be calculated from the equivalent mass.
Faraday’s second law of electrolysis : It states that, “When the same quantity of electricity is passed through solutions of different electrolytes connected in series, the weight of the substances produced at the electrodes are directly proportional to their equivalent mass.”
For example, for CuSO4 solution and AgNO3 solution connected in series, if the same quantity of electricity is passed, then
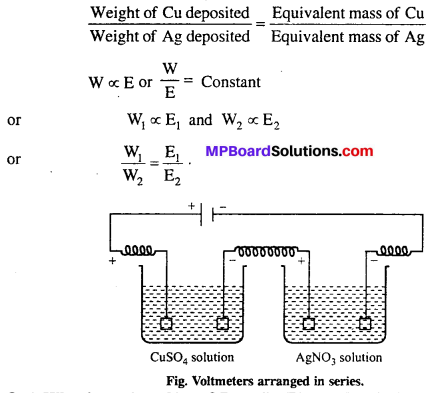
Question 4.
What is rusting of iron ? Describe Electrochemical theory of rusting.
Answer:
Corrosion: Process by which the layers of undesirable compounds are formed on the surface of a metal on its exposure to atmospheric condition are called corrosion. Rusting of iron is an example of corrosion, chemically it is Fe2O3xH2O.
Electrochemical theory of rusting :
Anode reaction : On one spot of iron sheet, oxidation takes place and this spot behaves as an anode.
![]()
The electrons which are released at this spot travel through the metal and reach another spot on the metal which acts as cathode. These electrons cause the reduction of oxygen in the presence of hydrogen ions (H+). H+ ions are formed due to decomposition of carbonic acid formed by dissolution of CO2 in H2O.
![]()
Question 5.
What is corrosion ? Write three factors affecting it and any three methods to prevent it.
Answer:
Corrosion : Process by which the layers of undesirable compounds are formed on the surface of a metal on its exposure to atmospheric conditions are called corrosion.
Factors affecting corrosion :
- Nature of metal: Reactive metal corrodes readily.
- Impurities in metal: Impure metal corrodes quickly to a greater extent.
- Environment: If environment around metal contains oxygens, carbon dioxide, moisture, salts and acidic gases like CO2, SO2, SO3 etc. then corrosion occurs quickly.
Methods to prevent corrosion :
1. Barrier protection : In this method, the surface of the metal is coated with paint, oil or grease. Due to this the surface of the metal remain unexposed to atmospheric conditions and hence corrosion is prevented. Surface of the metal can also be protected by :
(i) Coating metal surface with non-corroding metals like nickel and chromium is called electroplating.
(ii) Dipping iron article in molten metal like zinc. This process is called galvanization.
2. Sacrificial protection : The rusting of iron can be prevented by covering the iron with more electropositive metals like zinc. Zinc metal has more tendency to get oxidized as compared to iron. So, iron articles will not be harmed till the layer of zinc present on its surface hence zinc metal is called the sacrificial metal.
3. Antirust solution : The alkaline antirust solution are employed to prevent rusting, alkaline solution prevent the availability of H+ ions.
In this method, iron articles are dipped in alkaline sodium phosphate or chromate solution. Due to this an insoluble sticking film of iron phosphate is formed on the surface which prevents rusting.
![]()
Question 6.
Describe dry cell with labelled diagram.
Answer:
Dry cell: It is a primary cell based on Leclanche cell invented by G. Leclanche in 1868. In a primary cell, the electrode reactions cannot be reversed by an external source of electrical energy. In this cell, the cell reaction takes place only once i.e., this cell is not rechargeable.
It is generally used in torches, transistors, radios, calculators, tape recorders, etc. It consists of a hollow zinc cylinder which is filled with a paste of NH4Cl and a little ZnCl2. This paste is made with the help of water. The zinc cylinder acts as anode while cathode is a graphite rod (Carbon). The carbon rod is surrounded by a black paste of MnO2 and carbon powder. The zinc cylinder has an outer insulation of cardboard case.
Dry cells are sealed with wax or other material to protect the moisture from evaporation. When the electrodes are connected, the cell operates.
The electrode reactions are complex. Metallic Zn is oxidized to Zn2+ and the electrons liberated are left on the container. The reactions which take place at electrodes can be represented as :
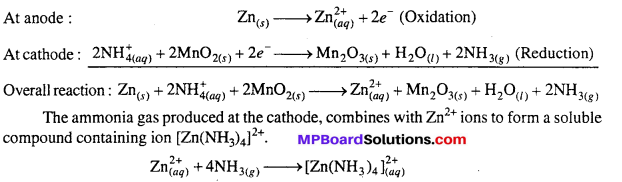
This reaction prevents polarization due to formation of ammonia. It also prevents the substantiaL increase of concentration of Zn2+ ions which would decrease the cell potential. This potential of dry cell is approximately 1.5 V.
Defect: Due to acidic nature of NH4Cl zinc container corrodes due to which holes develop through which the chemicals come out.
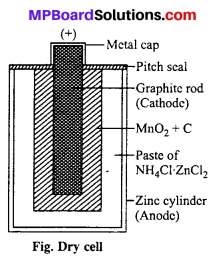
Nowadays, the cells are made leakage resistant. In it KOH is used in place of NH4Cl by which zinc does not corrode.
Question 7.
What is Kohlrausch law ? Give its two applications.
Answer:
Kohlrausch in 1875 gave a generalisation known as Kohlrausch’s law, “At infinite dilution when the dissociation of the electrolyte is complete, each ion makes a definite contribution towards molar conductance of the electrolyte irrespective of the nature of the other ion with which it is associated.”
Or
“The value of molar conductance at infinite dilution is given by the sum of the contributions of ions (cation and anion).”
![]()
Where, λ∞+ and λ∞– are ionic contributions or ionic conductances of cation and anion while v+ and v– are the number of cations and anions in the formula unit of electrolyte.
Applications of Kohlrausch’s law :
(i) Calculation of molar conductance at infinite dilution for weak electrolytes :
Molar conductance or equivalent conductance of weak electrolytes cannot be obtained graphically by extrapolation method, since these are feebly ionized. Kohlrausch’s law enables indirect evaluation in such cases. For example, molar conductances of acetic acid can be obtained from the knowledge of molar conductances at infinite dilution of HCl, CH3COONa and NaCl which are strong electrolytes.
From Kohlrausch’s law, it is clear that
![]()
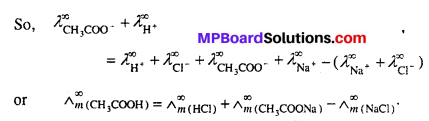
(ii) Determination of degree of dissociation :
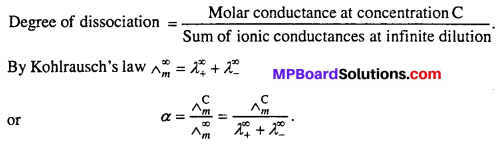
Question 8.
Draw a labelled diagram of Daniel cell and explain cell reaction.
Or,
Draw a labelled diagram of electrochemical cell and write cell reaction.
Answer:
Electrochemical cell: In the redox reactions, the transfer of electrons between oxidizing and reducing agents occurs through wire and thus chemical energy changes into electrical energy. The device on which chemical energy changes into electrical energy is called electro chemical cell. These are also known as galvanic or voltaic cells. Working of these cells can be understood with the example of Daniel cell.
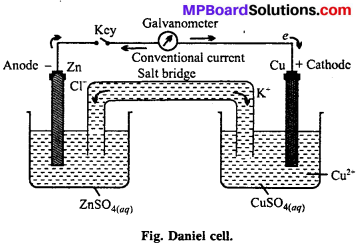
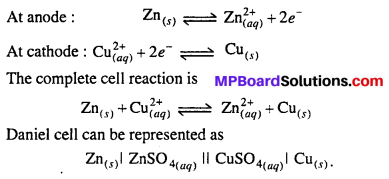
Daniel cell : In this cell, Zn rod is dipped in ZnSO4 solution and Cu rod in copper sulphate solution. Both solutions are connected through KC1 salt bridge. When Zn and Cu electrodes are connected by wire and galvanometer, flow of electrons from Zn to Cu occurs. Zinc atoms change into Zn2+ and electrons reach at Cu electrode, where Cu2+ changes into Cu metal and this copper deposits on electrode.
![]()
Electrochemistry Numerical Questions
Question 1.
On passing 5 ampere electric current for 30 minute through a container filled with AgNO3 10.07 gram silver is deposited, then determine the chemical equivalent of silver. If electrochemical equivalent of hydrogen is 0-00001036 then calculate the equivalent mass of Silver.
Solution:
Given : W = 10-07 gm, i = 5 ampere, t = 30 × 60 second
According to Faraday’s first law W = Zit
∴ 10.7 = Z × 30 × 60
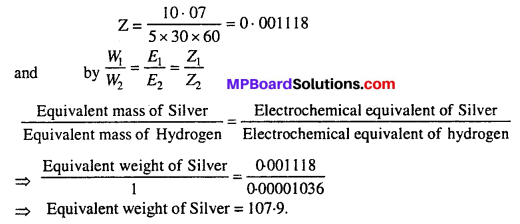
Question 2.
What are weak electrolytes ? Give one example. Find out molar conductivity of LiBr aqueous solution infinite dilution when joint conductance of Li+ ion and Br– ion are 38.7 Scm2 mol-1 and 78.40 Scm2 mol-1 respectively.
Solution:
Weak electrolytes : These are the substances which dissociate only to a small extent.
Examples: CH3COOH,NH4OH

Question 3.
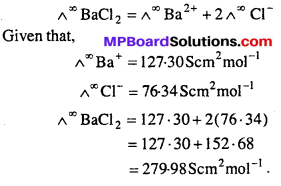
What are strong electrolytes ? Find out the molar conductivity of aqueous solution of BaCl2 at infinite dilution when ionic conductance of Ba+2 ion and Cl– ion are 127.30 Scm2 mol-1 and 76.34 Scm2 mol-1 respectively.
Solution:
Strong electrolytes : These are substances which dissociate almost completely into ions under all dilutions.
Examples: NaCl, HCl,CH3COONa
Question 4.
Calculate the molar conductance of Al2(SO4)3 if
![]()
![]()
Solution:
By the ionisation of Al2(SO4)3 solution
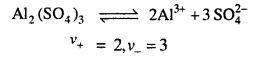

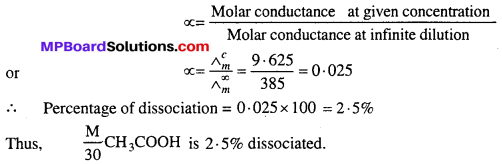
Question 5.
Molar conductivity of \(\frac { M }{ 30 } \) CH3COOH is 9.625 mho and molar conductance of CH3COOH at infinite dilution (Λ∞m) is 385 mho. Calculate the percentage of dissociation of \(\frac { M }{ 30 } \) CH3COOH.
Solution:
Degree of dissociation
Question 6.
Calculate molar conductance of acetic acid at infinite dilution from following values:

Solution:
From Kohlrausch’s law,