MP Board Class 12th Chemistry Solutions Chapter 13 Amines
Amines NCERT Intext Exercises
Question 1.
Classify the following amines as primary, secondary or tertiary:
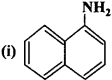
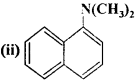
(iii) (C2H5)2CHNH2
(iv) (C2H5)2NH.
Answer:
- Primary (1°)
- Tertiary (3°)
- Primary (1°)
- Secondary (2°).
Question 2.
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N.
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines ?
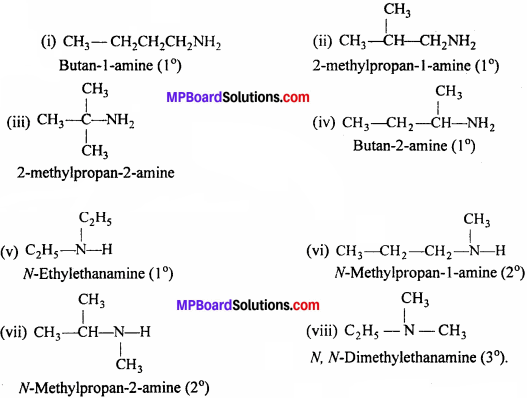
Isomerism:
(i)-(iv) and (vi)-(vii) are Position isomers, (v)-(vi) and (v)-(vii) are Metamers, (i)-(ii), (iii)-(iv) and (i)-(iii) are Chain isomers.
![]()
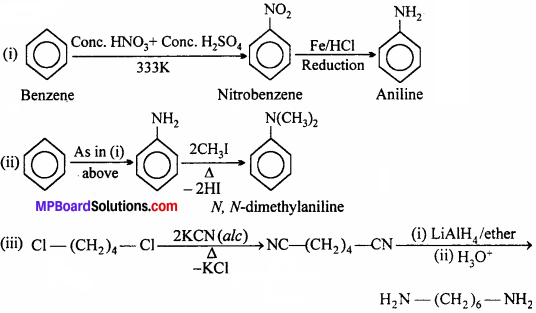
Question 3.
How will you convert:
(i) Benzene into aniline,
(ii) Benzene into N, N-dimethylaniline,
(iii) Cl – (CH2)4 – Cl into hexane -1, 6 – diamine.
Answer:
Question 4.
Arrange the following in increasing order of their basic strength:
- C2H5NH2, C62H5NH2,NH3, C6H5CH2NH2 and (C2H5)2NH
- C2H5NH2, (C2H2)2NH, (C2H5)3N, C6H5NH2
- CH3NH2, (CH3 )2NH, (CH3 )3N, C6H5NH2 , C6H5CH2NH2.
Answer:
- C6H5NH2 < NH3 < C6H5CH2NH2 < C2H5NH2 < (C2H5)2NH
- C6H5NH2 < C2H5NH2< (C2H5)3N < (C2H5)2NH
- C2H5NH2 < C6H5CH2NH2 < (CH3)3N < CH3NH2 < (CH3)2NH.
Question 5.
Complete the following acid-base reactions and name the products :
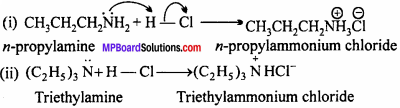
(i) CH3CH2CH2NH2 + HCl → ?
(ii) (C2HCl)3N + HCl → ?
Ans.
Question 6.
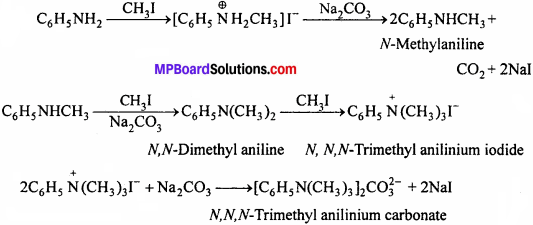
Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
Answer:
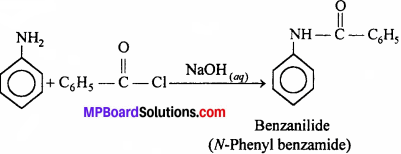
Question 7.
Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Answer:
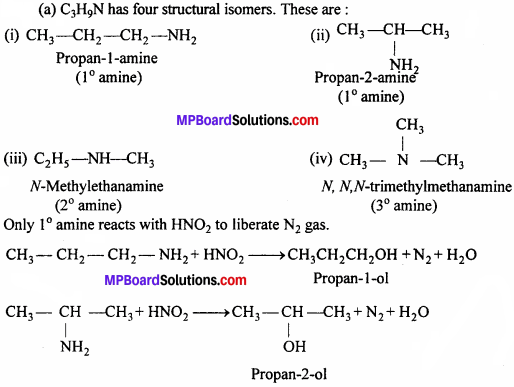
Question 8.
Write structures of different isomers corresponding to the molecular formula, C3H9N. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
Answer:
(a) C3H9N has four structural isomers.
These are:
Question 9.
Convert:
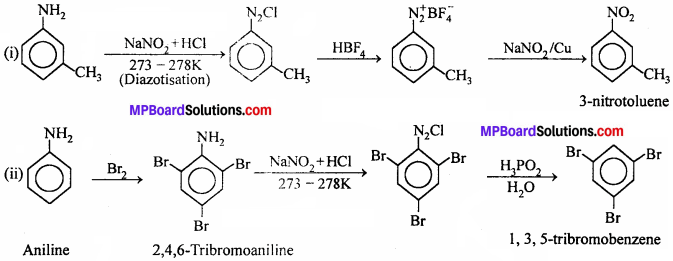
(i) 3-Methylaniline into 3 – nitrotoIuene
(ii) Aniline into 1,3, 5 – tribromobenzene
Answer:
Amines NCERT Text – Book Exercises
Question 1.
Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines:
- (CH3)2CHNH3
- CH3 (CH2 )2 NH 2
- CH3NHCH(CH3)2
- (CH3)3CNH2
- C6H5NHCH3
- (CH3CH2)3NCH3
- m – BrC6H4NH3.
Answer:
- Propan – 2 – amine (1°)
- Propan – 1 – amine (1 °)
- N – Methylpropan – 2 – amine (2°)
- 2 – MethyIpropan – 2 – amine (3°)
- N – Methylbenzenamine or A’-Methylaniline (2°)
- N – Ethyl – A – Methylethanamine (3°)
- 3 – Bromobenzenamine or 3-Bromoaniline (1°).
![]()
Question 2.
Give one chemical test to distinguish between the following pairs of compounds:
- Methylamine and dimethylamine,
- Secondary and tertiary amines,
- Ethylamine and aniline
- Aniline and benzylamine
- Aniline and N- methylaniline.
Answer:
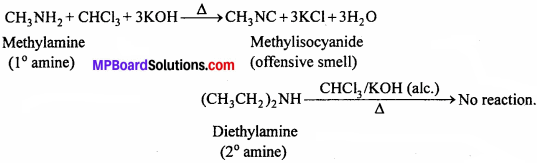
(i) By Carbylamine test:
Methylamine is a primary amine, therefore, it gives carbylamine test. In contrast, diethylamine is a secondary amine and hence does not give this test.
(ii) By Libermann Nitrosoamine test:
2° Amines on treatment with HNO2(generates in situ by the action of HCl on NaNO2) give yellow coloured oily N-nitrosoamine. For Example
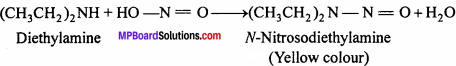
N-Nitrosodiethylamine on warming with phenol and cone. H2SO2 gives a green solu-tion which when made alkaline with aqueous NaOH turns deep blue and then red on dilu-tion. Tertiary amines do not give this test.
(iii) Azodye test:
It involves the reaction of any aromatic primary amine with HNO2 (NaNO2 + dil. HCl) at 273 – 278 K followed by treatment with an alkaline solution of ( β – naphthol) when a brilliant yellow, orange or red coloured dye is obtained
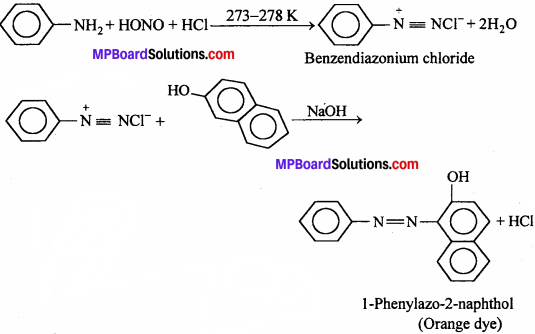
Aliphate primary amines under these conditions give a brisk evolution of N2 gas with the formation of priamary alcohols i.e. the solution remains clear.
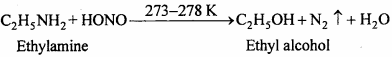
(iv) Aniline gives dye test (see, iii):
Benzyl amine reacts with nitrous acid to form benzyl alcohol and nitrogen gas (seen as bubbles).
C6H5CH2NH2 + NaOH → C6H5CH2OH + N2+ H2O
(v) These can be distinguished by carbylamine test:
Aniline being primary amines gives carbylamine test i.e. when heated with an alcoholic solution of KOH and CHCl2, it gives foul smell of phenylisocyanide.
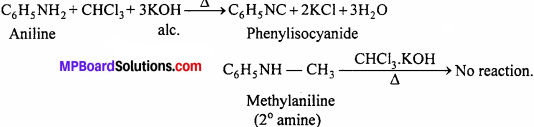
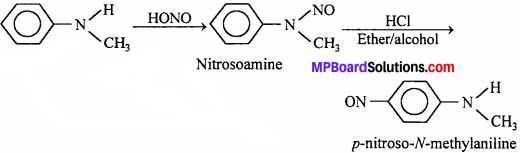
Methylaniline forms nitrosoamine (yellow oily liquid) on treatment with nitrous acid, which is stable at room temperature. However, on reaction with HCl in ether and alcohol, the nitroso (- NO) group migrates to para position.
Question 3.
Account for the following:
- pKb of aniline is more than that of methylamine.
- Ethylamine is soluble in water whereas aniline is not.
- Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
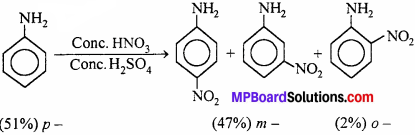
- Although amino group is O – and p – directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
- Aniline does not undergo Friedel- Crafts reaction.
- Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
- Gabriel phthalimide synthesis is preferred for synthesizing primary amines.
Answer:
(i) In aniline, the lone pair electrons on the N-atoms are delocalized over the benzene ring thereby decreasing the electron density on the N – atom. In contrast, in CH3NH2, + 1 effect of CH3 increases the electron density on the N-atom. Therefore, aniline is a weaker base than methylamine and hence its pKb value is higher than that of methylamine.
(ii) Ethylamine dissolves in water due to inter molecular H-bonding. In aniline, due to the large hydrophobic part, i.e. hydrocarbon part, the extent of H – bonding decreases considerably and hence, aniline is insoluble in water.
(iii) Methylamine being more basic than water, accepts a proton from water liberating OH– ions,
![]()
These OH– ions combine with Fe+3 ions present in H2O to form brown ppt. of hydrated ferric oxide.
FeCl3 → Fe+3+ 3Cl–
2Fe+3+ 6OH–→ 2Fe(OH)3 or Fe2O3.3H2O
Hydrates ferric oxide (Brown ppt.)
(iv) In strongly acidic medium (cone. HNO3/conc. H2SO4), most of the aniline gets protonated to form anilinium ion \(\left(\stackrel{\ominus}{\mathrm{N}} \mathrm{H}_{3}\right)\) which is m – directing and deactivating while – NH2 group in aniline is o, p – directing and activating. That is why besides the o, p – deriva- tives, significant amount of meta derivative is also formed.
(v) Aniline being a Lewis base reacts with Lewis acid AlCl3 to form a salt.
C6H5NH2+AlCl3 → C6H5 N⊖ H2 AlCl3⊖
Lewis base Lewis acid
Consequently, N of aniline acquires positive charge and thus acts a strong deactivating group for electrophilic substitution reaction. As a result, aniline does not undergo Friedel – Crafts reaction.
(vi) The diazonium salts of aromatic amines are more stable than those of aliphatic amines due to delocalization of the positive charge on the benzene ring.
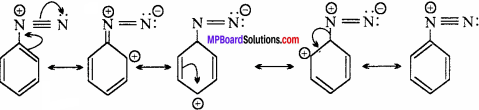
(vii) Gabriel phthalimide reaction gives pure 1° amines without any contamination of 2° and 3° amines. Therefore, it is preferred for synthesizing 1° amines.
Question 4.
Arrange the following:
(i) In decreasing order of the pKb values:
C6H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
(ii) In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2 ,(C2H5)2NH and CH3NH2
(iii) In increasing order of basic strength:
(a) Aniline, p-nitroaniline and p – toluidine
(b) C6H5NH2, C6H5NHCH3, C6H5CH2NH2
(iv) In decreasing order of basic strength in gas phase:
C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
(v) In increasing order of boiling point:
C6H5OH, (CH3)2NH, C2H5NH2
(vi) In increasing order of solubility in water:
C6H5NH2, (C2H5)2NH, C2H5NH2.
Answer:
(i) Due to delocalization of lone pair of electrons of the N-atom over the benzene ring, C6H5NH2 and C6H5NHCH3 are for less basic than C2H5NH2 and (C2H5)2 NH. Further, due to + I effect of the CH3 group, C6H5NHCH3 is slightly more basic than C6H5NH2.
Among C2H5NH2 and (C2H5)2NH, (C2H5)2NH is more basic than C2H5NH2 due to greater + 1 effect of the two C2H5groups. Combining all these facts, the relative basic strength of these four amines decreases in the order.(C2H2)2NH > C2H2NH2 > C6H5NHCH2> C2H5NH2
Since, a stronger base has lower pKb value, therefore pKb values decreases in the reverse order:
C6H5 NH2 > C6H5NHCH3 > C2H5NH2 > (C2H2)2NH
(ii) As explained in –
Answer:
(a) the relative basic strength of the amines decreases in the order:
(C2H5)2NH > C6H5NHCH3 > C6H5NH2
Among CH3NH2 and (C2H5)2NH, due to the greater + I effect of the two C2H5 groups, (C2H5)2 is more basic than CH3NH2. Thus, the basic strength of the four amines decreases in the order: (C2H5)2NH > CH3NH2> C6H5NHCH3 > C6H5NH2
(iii) (a) The electron-donating groups increases the basic strength of amines while the electron-withdrawing groups decreases the basic strength. Therefore, basicity increases in the order:
p-nitroaniline < aniline < p – toludine.
(b) In C6H5NH2 and C6H5NHCH3, N is directly attached to the benzene ring and thus the lone pair of electrons on the N-atom is delocalized over the benzene ring. Therefore, both C6H5NH2 and C6H5NHCH3 are weaker bases than C6H5C H2NH2. Furthers due to + I effect of CH3 group. C6H5NHCH3 is a stronger base than C6H5NH2.
The basic strength increases in the order:
C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2
(iv) Solvent effects, i.e. stabilization of the conjugate acids due to H-bonding are absent in the gas phase. In the gas phase, basic strength mainly depends upon the + I effect of the alkyl groups. Thus, basic strength in the gas phase decreases in the order:
(C6H5)3N > (C2H5)2NH > C2H5NH2> NH3
(v) Since, the electronegativity of ‘O’ is higher than that of N, alcohols form stronger H-bonds than amines. Further, the extent of H-bonding depends upon the number of H – atoms on the N-atoms. Thus, the inter molecular forces are in the order C2H5 – OH > C5H5NH2 > (CH3)2NH. Therefore, the b.pts of the given three compounds increases in the order:
(CH3)2NH < C2H5NH2 < C2H5OH
(vi) Solubility decreases with:
(a) Increase in molecular mass of amines due to increase in the size of the hydrophobic hydrocarbon part, and.
(b) Decrease in the number of H – atoms on the N-atom which undergo H-bonding.
Among the given compounds, C6H5NH2has the highest molecular mass of 93 followed by (C2H5)5NH with molecular mass of 73 while C2H5NH2 has the lowest molecular mass of 45. Thus, the solubility increases in the order in which molecular mass decreases i.e.,
C6H5NH2 < (C2H5)2NH < C2H5NH2
![]()
Question 5.
How will you convert:
- Ethanoic acid into methanamine
- Hexanenitrile into 1 – aminopentane
- Methanol to ethanoic acid
- Ethanamine into methanamine
- Ethanoic acid into propanoic acid
- Methanamine into ethanamine
- Nitromethane into dimethylamine
- Propanoic acid into ethanoic acid.
Answer:
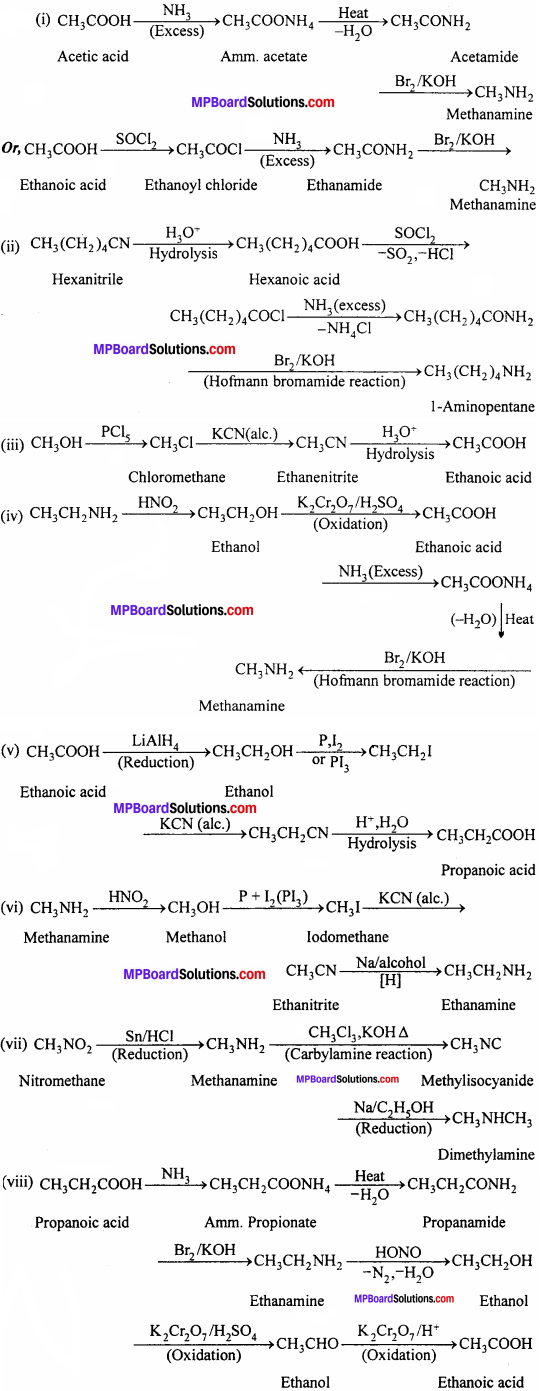
Question 6.
Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Answer:
Heinsberg’s test:
This is a excellent test for distinguishing between primary, secondary and tertiary amines. The amine is treated with benzene sulphonyl chloride (Hinsberg’s reagent) in presence of excess of aqueous potassium hydroxide solution. (Refer text for details)
Question 7.
Write short notes on the following:
- Carbylamine reaction
- Diazotisation
- Hofmann’s bromamide reaction
- Coupling reaction,
- Ammonolysis
- Acetylation
- Gabriel phthalimide synthesis.
Answer:
(i) Carbylamine reaction:
Primary aliphatic amine or aniline, when warmed with chloroform in presence of alcoholic KOH, gives iso cyanide or carbylamine, a compound with disagreeable odour. This reaction is known as Carbylamine reaction.
C6H5NH2 (aniline)+ CHCl3 + 3KOH → C6H5N \(\overrightarrow{=}\) C + 3KCl + H2O aniline phenyl isocyanide
(ii) Diazotisation:
By action of NaNO2 solution in ice cooled solution of aromatic amine formed inorganic acid, diazonium salts are obtained.
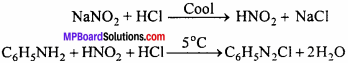
Diazonium salt contains diazo group (- N \(=\) N-) therefore, this process is called diazotization.
(iii) Hofmann’s bromamide reaction:
Primary aliphatic and aromatic amines can be prepared from amides by treatment with Br2 and KOH. The amine formed contains one carbon atom less than the parent amide. Therefore, this method is used for stepping down the series in organic conversion. Due to this reason it is also known as Hofmann degradation.
RCONH2 (Alkanamide ) + Br2 + 3NaOH > RNH2 (Alkanamine)+ 2NaBr+NaHCO3 + H2O
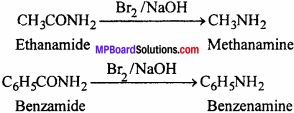
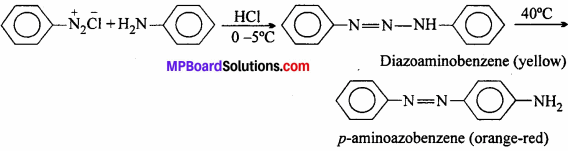
(iv) Coupling reaction:
Aniline reacts with diazonium chloride at ice cold temperature to give bright orange-red dye.
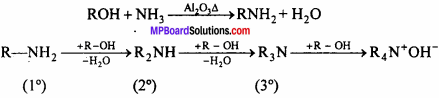
(v) Ammonoiysis:
It is a process of replacement of either halogen atom in alkyl halids (or aryl halides) or hydroxyl group in alcohols (or phenols) by amino group. The reagent used for ammonoiysis is alcoholic ammonia. Generally, a mixture of primary, secondary and tertiary Amine is fromed
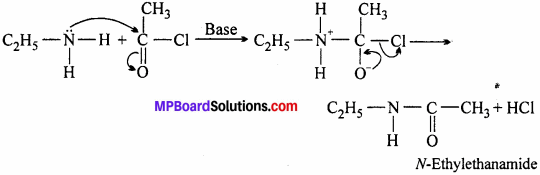
(vi) Acetylation:
Aliphatic and aromatic primary and secondary amines react with acid chlorides, anhydrides and esters by nucleophilic substitution reaction. This reaction is amine is formed. considered as replacement of hydrogen atom of — NH2 or >NH group by acyl group. This reaction is known as acylation.
The reaction is carried out in the presence of a base stronger than amine like pyridine, which removes HCl so formed and shift the equilibrium towards right hand side. The product obtained by acylation reaction is known as amides.
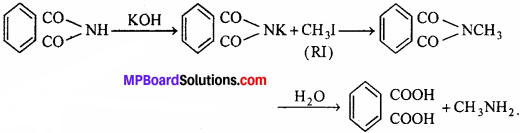
(vii) Gabriel phthalimide synthesis:
In this reaction, potassium phthalimide react with alkyl halide to form N-methyl phthalimide which on hydrolysis give primary amine
Question 8.
Accomplish the following conversions:
- Nitrobenzene to benzoic acid
- Benzene to m – bromophenol
- Benzoic acid to aniline
- Aniline to 2,4,6 – tribromofluorobenzene
- Benzyl chloride to 2 – phenylethanamine
- Chlorobenzene to p – chloroaniline
- Aniline to p – bromoaniline
- Benzamide to toluene
- Aniline to benzyl alcohol.
Answer:
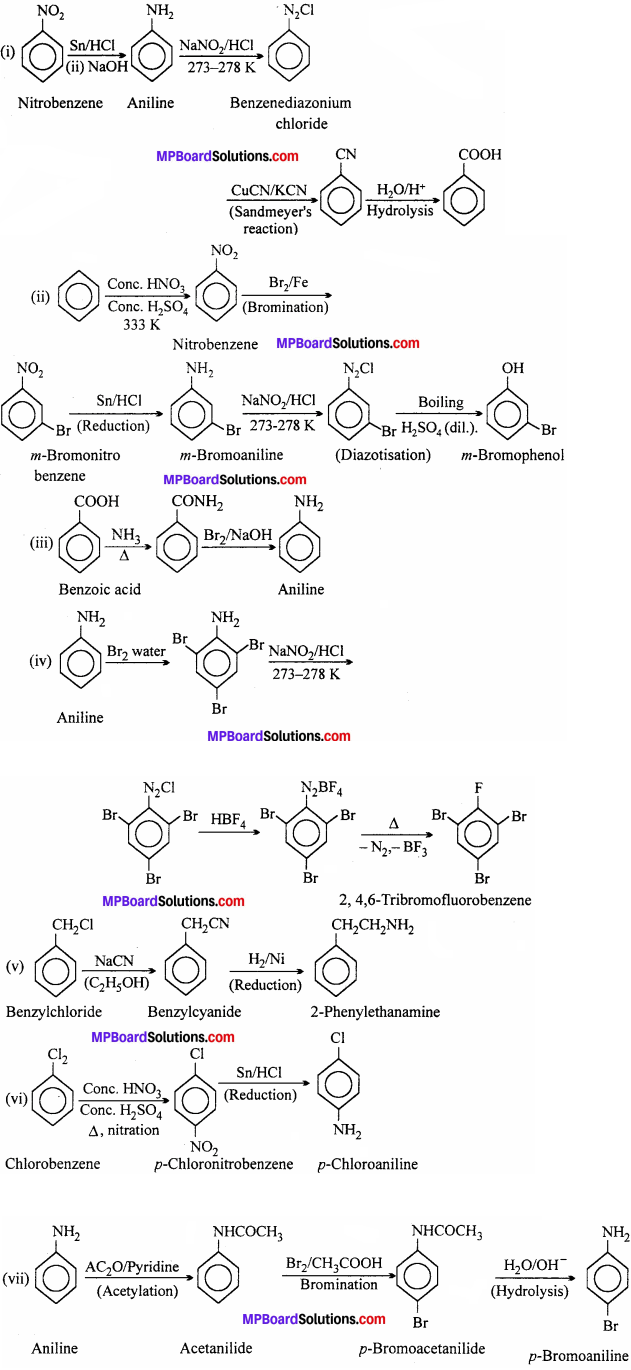
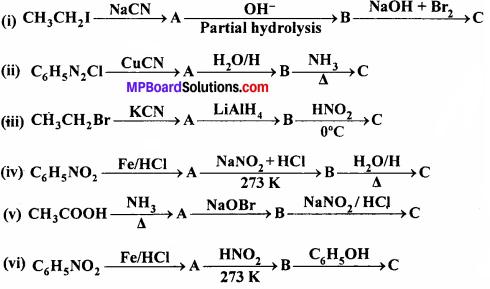
Question 9.
Give the structures of A, B and C in the following reactions:
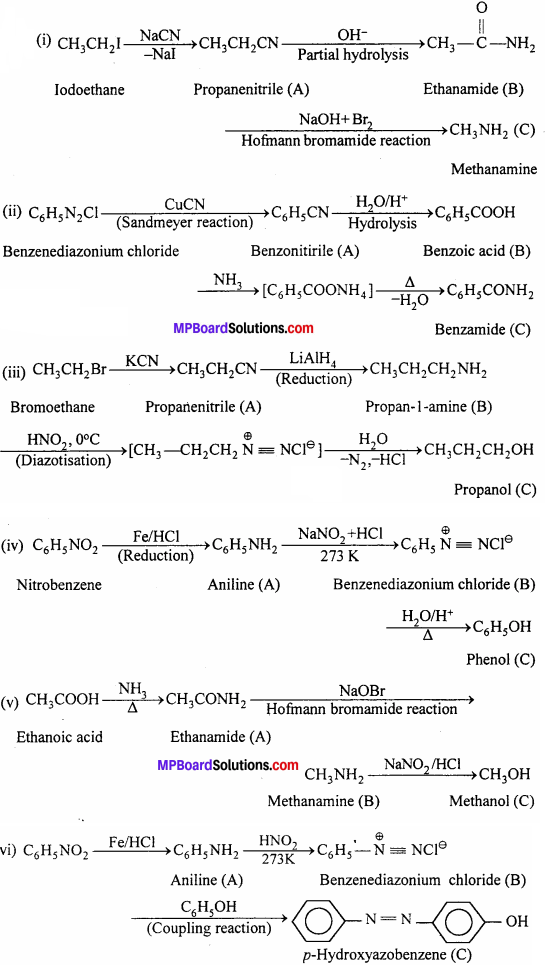
Answer:
Question 10.
An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Answer:
Structures of compounds ‘B’ and ‘C’:
(i) Since, ‘C’ is formed from ‘B’ on treatment with Br2 + KOH (i.e., Hofmann bromamide reaction), ‘B’ must be an amide and ‘C’ must be an amine. The only amine having the molecular formula C6H5NH2 benzene amine or aniline).
(ii) Since, ‘C’ is aniline, the amide from which it is formed must be benzamide (C6H5CONH2). Thus, compound ‘B’ is benzamide. The chemical equation showing the conversion of‘B’ to ‘C’ is,
![]()
Structure of compound ‘A’:
Since, ‘A’ on treatment with aqueous ammonia and heating forms benzamide ‘A’ must be benzoic acid.

Question 11.
Complete the following reactions:
(i) C6H5NH2 + CHCl3 + (afc.)KOH →
(ii) C6H5N2Cl + H3PO2+H2O →
(iii) C6H5NH2+H2SO4(Conc.) →
(iv) C6H5N2C1 + C2H5OH →
(v) C6H5NH2 + Br2 →
(vi) C6H5NH2+ (CH3CO)2O →
![]()
Answer:
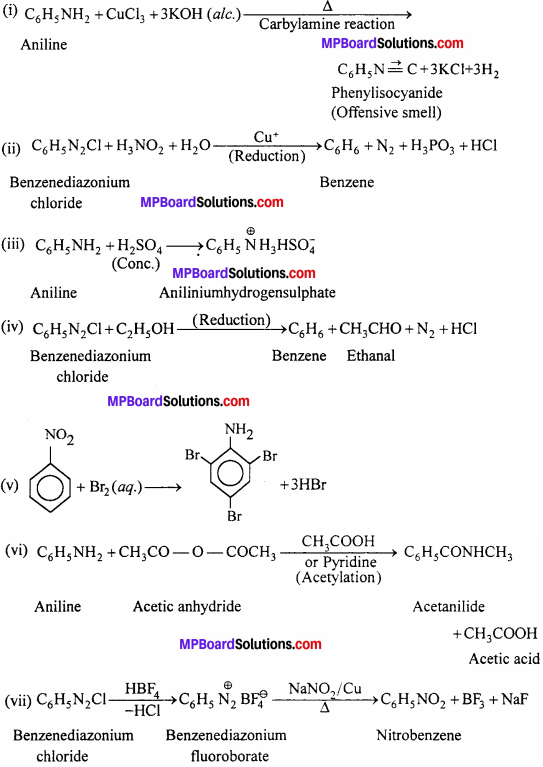
Question 12.
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis ?
Answer:
Gabriel Phthalimide reaction occurs through the nucleophilic attack by the phthalimide anion on the organic halogen compound.
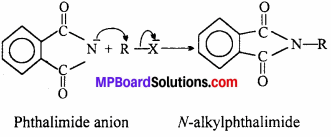
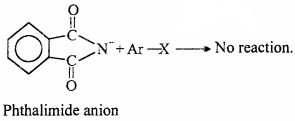
Since, aryl halide do not undergo nucleophilic substitution reactions easily aromatic primary amines cannot be prepared by Gabriel phthalimide.
Question 13.
Write the reactions of:
- Aromatic
- Aliphatic primary amines with nitrous acid.
Answer:
(i) Aromatic primary amines react HNO2 at 273 – 278 K to form aromatic diazonium salts.
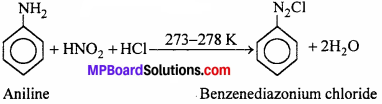
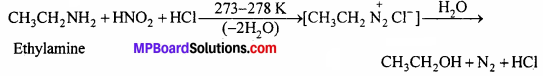
(ii) Aliphatic primary amines also react with HNO2 at 273-278 K to form aliphatic diazonium salt. But these are unstable even at this low temperature and thus decompose readily to form a mixture of compounds consisting of alkyl chlorides, alkenes and alcohols, out of which alcohols generally predominate.
Question 14.
Give plausible explanation for each of the following:
- Why are amines less acidic than alcohols of comparable molecular masses ?
- Why do primary amines have higher boiling point than tertiary amines ?
- Why are aliphatic amines stronger bases than aromatic amines ?
Answer:
(i) Amines lose a proton to form an amide ion while alcohol lose a proton to give an alkoxide ion.
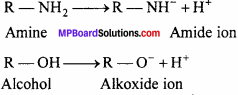
Since, ‘O’ is more electronegative than N, RO– can accommodate the -ve charge more easily than the RNH– ion. In
other words, RO- is more stable than RNH– Thus, alcohols are more acidic than amines or amines are less acidic than alcohols.
(ii) Primary amines (R—NH2) have two hydrogen atom on the N atom and so, they undergo extensive intermolecular H-bonding.
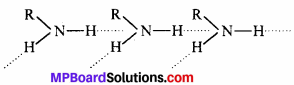
Tertiary amines (R3N) do not have hydrogen atoms on the N-atom and therefore, they do not undergo H-bonding. As a result, primary amines have higher boiling point than tertiary amines of comparable molecular mass. For example, Boiling point of H-butylamine (35 IK) is much higher than that of tertiary butylamine (b.p. 319 K).
![]()
Amines Other Important Exercises
Amines Objective Type Questions
Question 1.
Choose the correct answer:
Question 1.
Aniline reacts with cold nitrous acid (NaNO2 + HCl) and gives:
(a) C6H5OH
(b) C6H5N2Cl
(C) C6H5NO2
(d) C6H5Cl.
Answer:
(b) C6H5N2Cl
Question 2.
A nitrogen-containing compound, on heating with chloroform and alcoholic KOH give vapours of disagreeable odour. The compound can be:
(a) Nitrobenzene
(b) Benzamide
(c) N-N, dimethylaniline
(d) Aniline.
Answer:
(d) Aniline.
Question 3.
Ethylamine reacts with nitrous acid to form:
(a) Ammonia
(b) Nitrous oxide
(c) Ethane
(d) Nitrogen.
Answer:
(d) Nitrogen.
Question 4.
A compound which gives oily nitrosoamine with nitrous acid at low temperature:
(a) Methyl amine
(b) Dimethyl amine
(c) Trimethyl amine
(d) Triethyl amine.
Answer:
(b) Dimethyl amine
Question 5.
Which of the following has strongest basic character:
(a) C6H5NH2
(b) (CH3)2NH
(C) (CH3)3N
(d) NH3.
Answer:
(b) (CH3)2NH
![]()
Question 6.
Benzene diazonium chloride gives on hydrolysis:
(a) Chlorobenzene
(b) Phenol
(c) Alcohol
(d) Benzene.
Answer:
(b) Phenol
Question 7.
In the reaction C6H5CHO + C6H5NH2 → C6H5N = CHC6H5 + H2O + C6H5N = CHC6H5 is known as:
(a) Aldol
(b) Schiff’s reagent
(c) Schiffs base
(d) Benedict reagent.
Answer:
(c) Schiffs base
Question 8.
Nitrobenzene gives N-phenyl hydroxyl amine when it reacts with:
(a) Sn/HCl
(b) C6H5CH2NH-CH3
(c) Zn / NaOH
(d) Zn / NH4Cl.
Answer:
(c) Zn / NaOH
Question 9.
Which of the mixture when reacts with ale. KOH known as Carbyl amine reaction:
(a) Chloroform and Ag powder
(b) Trihalogenated methane and primary amine
(c) Alkyl trihalide and primary amine
(d) Alkyl cyanide and primary amine.
Answer:
(b) Trihalogenated methane and primary amine
Question 10.
Which of the following gas is responsible for Bhopal gas tragedy in 1984:
(a) CH3 – N = C = O
(b) CH3-C=N = S
(c) CHCl3
(d) C6H5COCl.
Answer:
(a) CH3 – N = C = O
Question 11.
Oil of Mirbane is:
(a) Aniline
(b) Nitrobenzene
(c) p-nitroaniline
(d) p-aminoazobenzene.
Answer:
(b) Nitrobenzene
![]()
Question 12.
The product of mustard oil reaction is:
(a) Alkyl isothiocyanate
(b) Dithiocarbonamide
(c) Dithioethyl acetate
(d) Thioether.
Answer:
(a) Alkyl isothiocyanate
Question 13.
Aniline is purified by:
(a) Steam distillation
(b) Vacuum distillation
(b) Simple distillation
(d) Solvent extraction.
Answer:
(a) Steam distillation
Question 14.
Amine which will not react with Acetyl chloride, is:
(a) CH3NH2
(b) (CH3)2NH
(c) (CH3)2N
(d) None of these.
Answer:
(c) (CH3)2N
Question 15.
![]()
(a) Gattermann’s reaction
(b) Sandmayer’s reaction
(c) Wurtz’s reaction
(d) Frankland’s reaction.
Answer:
(b) Sandmayer’s reaction
Question 2.
Fill in the blanks:
- Aromatic amines are …………… in water.
- Amines are benzolyate in presence of NaOH. This reaction is called ……………
- By reacting with nitrous acid 1° amine form alcohol and 2° amine form ……………
- Reaction of 2° amine with nitrous acid represent ……………
- Aniline on sulphonation with sulphuric acid forms ……………
- With metal (Transition metals) ions amine establish co-ordination and form ……………
- By reduction, cyanide forms …………… and isocyanide forms ……………
- Benzoic acid reacts with hydrozoic acid to form ……………
- All aliphatic amines are more …………… than ammonia.
- 1° and 2° amine react with Grignard reagent to form ……………
- Basic nature of amine is due to the presence of …………… on nitrogen atom.
- Primary amines on heating with …………… and …………… form alkyl isocyanides.
- C6H5-COOH + …………… C6H5NH2 + N2 + CO2.
- Mixture of T.N.T. and ammonium nitrate is known as ……………
- On reacting aniline with HCl and NaNO2 at 0°C temperature benzene diazoniu chloride is formed. This is called…………… reaction.
- Ethyl amine is a …………… base than ammonia.
- Trinitrotoluene is an …………… compound.
- On heating alkyl isocyanide to 250°C …………… is formed.
Answer:
- Insoluble
- Schotten Baumann
- Nitrosamine
- Libermann nitroso test
- Sulphanilic acid
- Complex ion
- Primary amine, secondary amine
- Aniline
- Basic
- Alkane
- lone electron pair
- Chloroform and caustic soda
- N3H
- amytoi
- diazotisation
- stronger
- explosive
- Alkyl cyanide.
![]()
Question 3.
Match the followings:
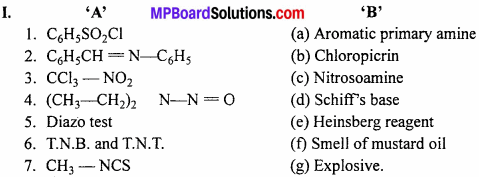
- (c) Nitrosoamine
- (d) SchifFs base
- (b) Chloropicrin
- (e) Heinsberg reagent
- (a) Aromatic primary amine
- (g) Explosive.
- (f) Smell of mustard oil

Answer:
- (e) C6H5SO2Cl
- (d) Explosive
- (a) Chloropicrin
- (c) C6H5N2Cl
- (b) Alcohol
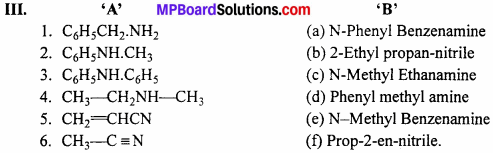
- (d) Phenyl methyl amine
- (e) N-Methyl Benzenamine
- (a) N-Phenyl Benzenamine
- (c) N-Methyl Ethanamine
- (f) Prop-2-en-nitrile.
- (b) 2-Ethyl propan-nitrile
Question 4.
Answer in one word/sentence:
- Why aniline turns blackish brown in open air ?
- Tertiary amine does not acetanilised, why ?
- Which isomer of C3H9N is least basic and having lowest b.p. ?
- Which amine gives diazotization reaction ?
- The compound obtained when primary aromatic amine when heated with CHCl3 and ale. KOH.
- Secondary amine can be identified by.
- What is nitrating mixture ?
- Nitrobenzene is known as.
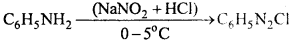
- Write the nature of amine.
- Primary nitroalkane reacts with nitrous acid to form which compound.
- Reagent used for the separation of Primary Secondary and Tertiary amine.
- What do 1° and 2° amine form on reacting with phosgene ?
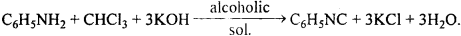
- What is obtained by reacting amines with chloroform?
- In which form is amine used in organic synthesis ?
- What does Ethylamine form on oxidation in the presence of KMnO4 ?
- On adding Br2 water in aqueous solution of C6H5NH2which precipitate is obtained ?
- Which amine is obtained by the reduction of cyanide in the presence of Pt or Ni ?
- Write the formula of X:
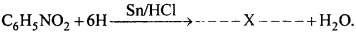
- Write the name of reaction:
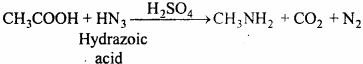
- What is the name of reaction for preparation of methyl isocyanide ?
Answer:
- Aniline oxidizes by air
- Active hydrogen is absent
- Tertiary amine
- All primary aromatic amine
- Phenyl isocyanide
- By Libermann test
- Cone. HNO3 and cone. H2SO4
- Oil of Mirbane
- Diazotisation
- Nitrolic acid
- Basic
- Heinsberg reagent
- Substituted urea
- Carbylamine reaction
- Alkyl isocyanide
- Reagent
- Aldehyde
- Symmetrical tribromoaniline
- 1° amine
- C6H5NH2 (Aniline)
- Schmidt reaction
- Carbylamine reaction.
![]()
Amines Short Answer Type Questions
Question 1.
Aniline is insoluble in water but soluble in HCl. Explain.
Answer:
Being basic nature, aniline forms soluble salts with strong acids like HCl while with water no such salt is formed. Therefore, aniline is insoluble in water but soluble in HCl.
C6H5NH2 + HCl → \(\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{3}^{+}\right] \mathrm{Cl}^{-}\)Aniline hydrochloride
Question 2.
Write a short note on Schotten Baumann reaction.
Answer:
Aromatic acid chloride reacts with phenol and aniline in presence of aqueous NaOH or pyridine. The reaction is known as Schotten Baumann reaction.
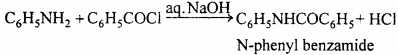
Question 3.
What is ‘aniline black’ ? Write its two uses.
Answer:
When aniline is oxidised by acidic KMnO4, a black substance is formed which is known as aniline black.
Uses:
- In manufacturing of black colour.
- In manufacturing of useful compounds.
Question 4.
(i) Boiling point of alkyl cyanides is higher than alkyl halides of nearly same molecular mass. Why ?
(ii) B.P. and M.P. of isocyanides are less than isomeric cyanide compounds. Why ?
Answer:
(i) Cyanide group (- C \(\cong\) N) is polar. Thus dipole moment of alkyl cyanide is higher due to which intermolecular force between them is higher. As a result boiling point of alkyl cyanide is higher than alkyl halides of nearly same molecular mass.
(ii) Isocyanide compounds are less polar than isomeric cyanides. Therefore, the boiling points and melting points of isocyanides are less than cyanides.
Question 5.
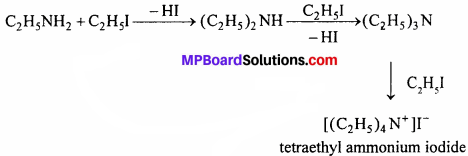
Complete the following reactions:
(a) C2H5I + H2N – C2H5 →
(b)CH2NH2 + (NaNO2 + HC1) →
Answer:
(a) This reaction is alkylation and ultimate product quartemary ammonium salt is formed
(b) CH3NH2 + (NaNO2 + HCl) → CH3OH + N2 + H2O
Question 6.
Ethyl amine is more basic than ammonia, why ?
Answer:
The value of Ka = 4.5 x 10-4 for ethyl amine and for ammonia it is 1.8 x 10-5. Larger is the Kb value, more basic is the amine and vice-versa. In ethyl amine the availabil-ity of lone pair of electrons on nitrogen atom increases due to the +1 inductive effect of the ethyl group. Hence, this lone pair of electrons can easily accept a proton. This explains why ethyl amine is more basic than ammonia.
![]()
Question 7.
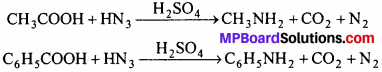
Write short notes on:
- Schmidt reaction
- Mustard oil reaction.
Answer:
(i) Schmidt reaction:
When hydrazoic acid dissolved in chloroform or benzene, react with mono carboxylic acid in presence of H2SO4 at 55°C, primary amine is obtained.
(ii) Mustard oil reaction:
When aliphatic primary amine is heated with carbon
disulphide and HgCl2 alkyl isothiocyanate is formed, which has smell like mustard oil. Therefore, this reaction is called mustard oil reaction.
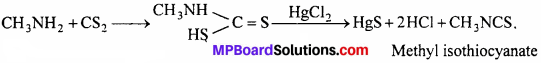
Question 9.
Give a Heinsberg method to identify primary, secondary and tertiary amines.
Answer:
Heinsberg method:
This method is capable to differentiate primary, secondary and tertiary amines. Amines are heated with benzene sulphonyl chloride (Heinsberg reagent) and various product are obtained.
1. Primary amine:
These form sulphonamide which are soluble in KOH.
C6H5SO2Cl + R – NH2 → C6H5SO2(NHRN – alkyl benzene sulphonamide) + HCl
2. Secondary amine:
Secondary amine also form sulphonamide which are insoluble in KOH.
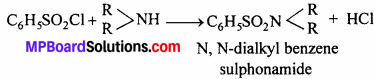
3. Tertiary amine : Tertiary amine does not react.
C6H5SO2Cl + R3N → No reaction
Question 9.
What is the difference between Alkyl nitrite and nitroalkane ?
Answer:
Nitrous acid exist in two isomeric forms:
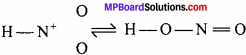
Thus, two alkyl derivatives of nitrous acid are formed.
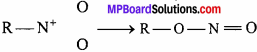
Thus, it is clear that in nitroalkane, alkyl radical is linked to nitrogen, whereas in alkylnitrite alkyl radical is linked to oxygen atom. Alkyl nitrite is ester whereas nitroalkane is the derivative of paraffin.
Question 10.
Write only chemical equation for the following conversions:
(i) Conversion of methyl cyanide into C6H5NH2.
(ii) Conversion of C6H5NH2 into chlorobenzene.
Answer:
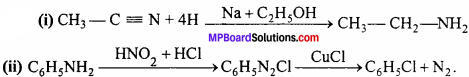
Question 11.
Differentiate between primary, secondary and tertiary nitroalkane.
Answer:
Primary, secondary and tertiary nitroalkanes can be differentiated on the basis of following reaction:
(i) Primary nitroalkanes react with nitrous acid forming nitrolic acid which is soluble in NaOH or KOH forming red colour.
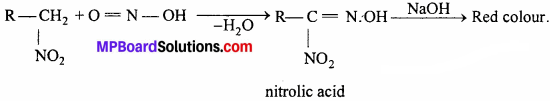
(ii) Secondary nitroalkane reacts with nitrous acid forming pseudonitrole which gives blue colour with NaOH.
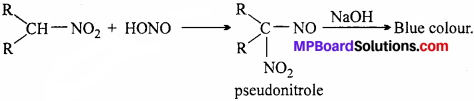
(iii) Tertiary nitroalkane does not react with nitrous acid because it does not have hydrogen atom.
Question 12.
Write the points of difference between Ethyl amine and Aniline.
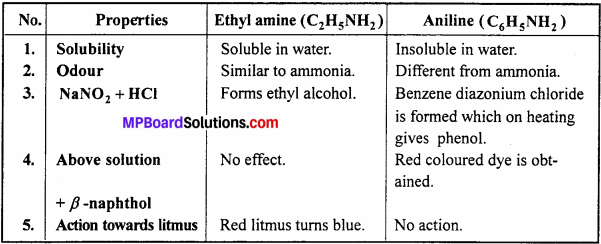
Answer:
Differences between Ethyl amine and Aniline:
Question 13.
Why can aromatic primary amine not be synthesized by Gabriel phthalimide synthesis.
Answer:
In Gabriel Phthalimide reaction potassium salt of phthalimide is formed. It easily reacts with alkyl halide and forms the related alkyl derivative.
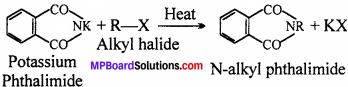
But aryl halide do not react with potassium phthalimide because in haloarene (aryl halide) due to partial double bond character breaking of C – X bond is difficult. Thus, aromatic amine can not be prepared by Gabriel Phthalimide synthesis.
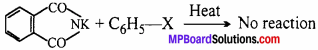
Question 14.
Write any four points to differentiate between Ethyl nitrite and nitro ethane.
Answer:
Difference between nitro ethane and ethyl nitrite.
1. Structure:

2. Boiling Point:
Boiling point of nitroethane is higher than Ethyl nitrite.
3. Reduction:
Nitroethane forms primary amine and ethyl nitrite forms ammonia.
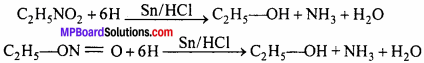
4. Hydrolysis:
Nitroethane does not form alcohol with NaOH whereas Ethyl nitrite forms alcohol with NaOH.
C2H5 – O – N + NaOH → C2H5OH + NaNO2
Question 15.
What is Mendius reaction ?
Ans. Reduction of alkyl cyanides by sodium and alcohols yield primary amine. This reaction is called Mendius reaction.
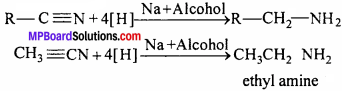
Question 16.
How will you obtain:
- Ethyl amine from methyl cyanide
- Methyl amine from acid amide
- Ethyl amine from ethyl alcohol
- Ethyl amine from methyl amine.
Answer:
1. Ethyl amine from methyl cyanide:
![]()
2. Methyl amine from acid-amide:
CH3CONH2 + Br2 + 4KOH → CH3 – NH2 + 2KBr + K2CO3 + 2H2O
3. Ethyl amine from ethylimg
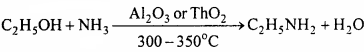
4. Ethyl amine from methyl amine:
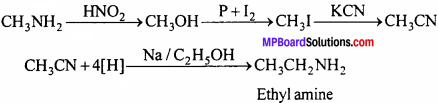
Question 17.
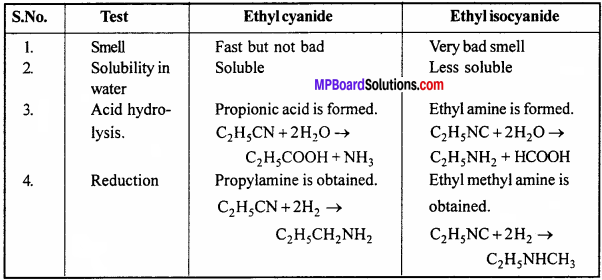
Write any four difference between ethyl cyanide and ethyl iso cyanide.
Answer:
Difference between ethyl cyanide and ethyl iso cyanide:
Question 18.
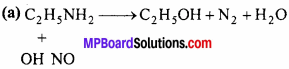
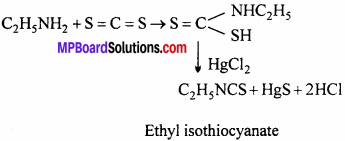
Give reaction of ethyl amine with following:
(a) HNO2
(b) C6H5COCl
(C) CS2.
Answer:
(a) HNO2
(b) N-ethyl acetamide is formed
C2H5NH2 + CH3COCl → CH3CONHC2H5 + HCl
(c) Ethyl isothiocyanate is formed. This reaction is also known as mustard oil reaction.
Question 19.
Why aniline is less basic than ethyl amine ?
Answer:
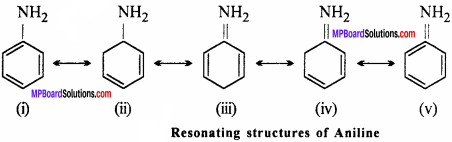
Aniline is less basic than ethyl amine as due to resonance of benzene nucleus, the lone pair of electron of nitrogen atom is attracted towards nucleus and gets delocalised in the ring. Thus, electron pair is liberated with difficulty in aniline than ethyl amine. Hence, its basic property is less than ethyl amine. The delocalisation of electron as a result of resonance is shown as follows:
Question 20.
Boiling point of aniline is higher than the hydrocarbons of comparable molecular masses, but lower than corresponding alcohols and carboxylic acids. Clarify the statement
Answer:
Primary amines are polar and in them dipolar [Nδ- – Nδ+] bond is present, in which molecules of amines are associated by hydrogen bond. That is why their boiling point is higher than alkanes of comparable molecular masses.
Due to lesser electronegativity of nitrogen than oxygen, association in alcohols and carboxylic acids occur due to strong H – bonds. Whereas in amines association is due to weak H — bond. Therefore, boiling and melting point of amines is lower than corresponding alcohols and carboxylic acids.
Question 21.
Explain nitration of Aniline.
Answer:
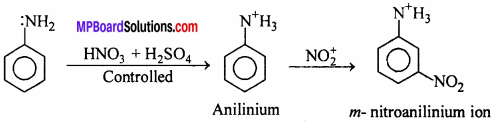
Direct nitration of aniline is not possible because NH3 group gets oxidized by HNO3 but on nitrating under
controlled conditions instead of o- and p- place m – nitroderivative is formed. In acidic medium due to protonation
of — NH2 group, electron attracting tendency of NH3+ formed increases by which instead of o- or p- directing Anilinium ion acts as m — directing.
Question 22.
Why is aniline acetylated before nitration ? Give necessary equation.
Or,
Direct nitration of aniline is not possible. Explain and how it is nitrated?
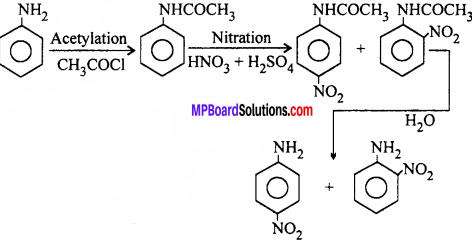
Answer:
Direct nitration of aniline is not possible because amino group of aniline is oxidised by nitric acid. So for nitration of aniline firstly amino group (- NH2) is protected by acetylation and then nitrated.
Question 23.
How will you obtain: (Give only equations)
- Methyl amine from Ethyl amine.
- Phenol from Aniline.
- Ethyl amine from methyl amine.
Answer:
1. Methyl amine from Ethyl amine:
2. Phenol from Aniline:
3. Ethyl amine from methyl amine:
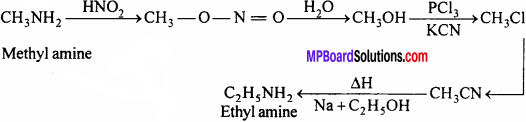
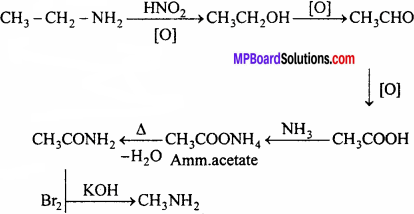
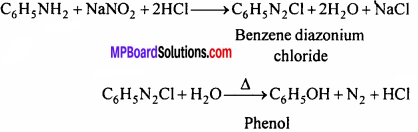
Question 24.
Explain the following:
- Preparation of pure amine is difficult by the ammonolysis of alkyl halide.
- Methyl amine reacts with FeCl3 in water to give precipitate of ferric hydroxide.
- AgCl is soluble in methyl amine.
Answer:
1. By the ammonolysis of alkyl halide a mixture of primary, secondary and tertiary amine is obtained.
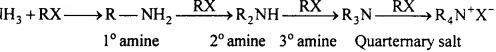
Separation of these amines is difficult; therefore it is difficult to obtain pure amine by ammonolysis of alkyl halide.
2. Methyl amine releases OH– ion in water which reacts with FeCl3 as follows to give precipitate of Fe(OH)3.
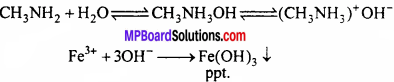
3. Methyl amine forms a soluble complex with AgCl and this way AgCl is soluble in methyl amine.
AgCl + 2CH3NH2 → [Ag(CH3NH2 )2 ]+ Cl– Soluble complex
Amines Long Answer Type Questions
Question 1.
Write reduction reactions of nitro-benzene:
(a) In acid medium
(b) Neutral medium
(c) In basic medium.
Or,
Describe reduction reactions of nitro-benzene in different conditions.
Answer:
Reduction of Nitro-benzene:
Nitro-benzene is readily reduced. This gives different compounds under different conditions depending upon the pH of the medium and the nature of the reducing agent. The reduction takes place in three steps:
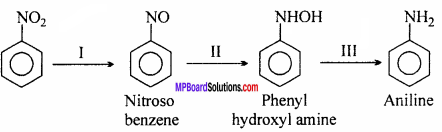
(a) Acidic medium:
When reduced with Sn + HCl or Fe + HCl, gives aniline.
![]()
(b) Neutral medium:
When reduced with aluminium mercury couple or zinc dust and ammonium chloride, phenyl hydroxyl amine is formed.
![]()
(c) In basic medium (Alkaline medium):
(i) Reduction with alkaline sodium arsenite:
Azoxy benzene is formed.
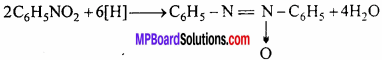
(ii) Reduction with zinc dust and caustic soda:
Hydrozo benzene is formed.
![]()
Question 2.
How will you obtain the following from aniline ? Give only chemical equation:
(a) Phenol
(b) Methane
(c) Tribromoaniline
(d) Phenylisocvanide.
Answer:
(a) Phenol:
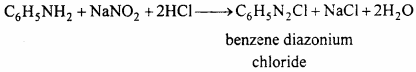
![]()
(b) Methane:
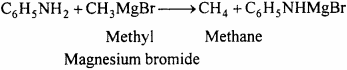
(c) Tribromoaniline:
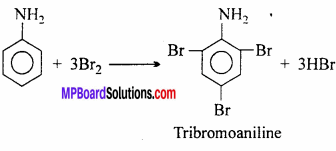
By treating aniline with bromine water, yellow coloured 2,4,6 – tribromoaniline is formed.
(d) Phenylisocyanide:
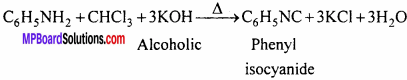
Question 3.
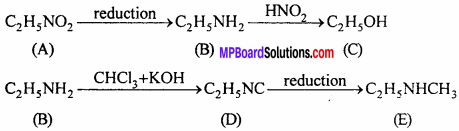
An organic compound [A] having molecular formula C2H5O2 on reduction gives compound [B] which react with HNO2 and form compound [C]. Compound [B] on reaction with chloroform alcoholic KOH, change into bad smelling compound [D]. Which on reduction forms amine [E]. Which formula will you assign for compound [A], [B], [C], [D] and [E] ? Explain reactions also.
Answer:
[A] → C2H5NO2 (Nitro ethane)
[B] → C2H5NH2(Ethyl amine)
[C] → C2H5OH (Ethanol)
[D] → C2H5N = C (Ethyl iso cyanide)
[E] → C2H5NHCH3(N-methyl amino ethane)
Reactions:
Question 4.
How will you obtain the following:
(i) Sulphanilic acid from Aniline
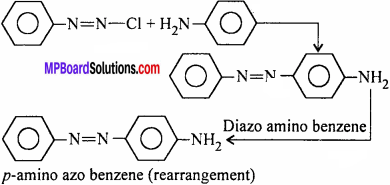
(ii) p-amino azo benzene from aniline.
Answer:
(i) Sulphanilic acid from Aniline
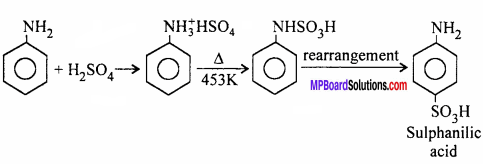
(ii) p-amino azo benzene from aniline.
Question 5.
What happens when:
(a) Ammonium acetate is made to react with alumina at 500°C.
(b) Methyl cyanide is subjected to alkaline hydrolysis.
(c) Benzamide is treated with P2Os.
(d) H2 gas is passed through phenyl isocyanide in presence of Ni.
(e) Methyl isocyanide is heated at 250°C.
Answer:
(a) Methyl cyanide is formed.
![]()
(b) Ammonia gas is formed.
![]()
(c) Phenyl cyanide is formed.
![]()
(d) N-methyl aniline is formed.
![]()
(e) Methyl cyanide is formed.
![]()
Question 6.
How is primary amine obtained from nitro-alkane ? Give equation.
(ii) How is primary alcohol obtained from primary amine ? Give equation.
(iii) Among N-propyl amine and isopropyl amine which is more basic ?
Answer:
(i) Nitro-alkane is reduced by LiAlH4 or by H2 in presence of Ni or Pt to obtain primary amine.
![]()
(ii) Primary aliphatic amine react with nitrous acid to form primary alcohol.
C2H5NH2 + HONO → C2H5OH + N2 + H2O
(iii) Normal propyl amine is more basic.
Question 7.
How will you obtain the following (Give only equation):
(a) Ethyl amine from nitroethane,
(b) N-methyl hydroxyl amine from nitroethane,
(c) Chloropicrin from nitromethane,
(d) Nitrobenzene from benzene diazonium chloride,
(e) Trinitrobenzene (T.N.B.)from nitrobenzene.
Answer:
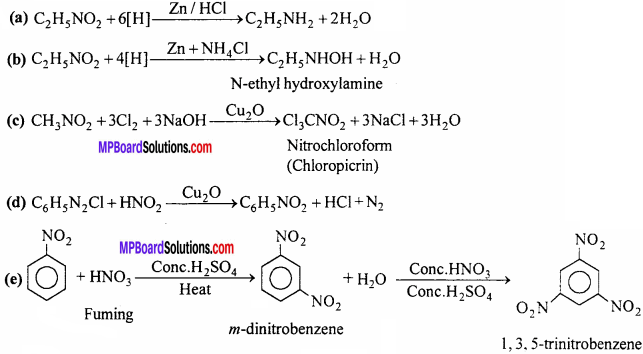
Question 8.
Explain the laboratory method of preparation of ethyl amine under the following heads:
(a) Procedure
(b) Equation of reaction
(c) Diagram
(d) Physical properties.
Answer:
Laboratory method of preparation of ethyl amine: In laboratory ethyl amine is prepared by Hofmann Bromoamide reaction. In this process reaction of propanamide with bromine and caustic potash solution takes place. All steps in the process are following:
C2H5CONH2 + Br2 → C2H5CONHBr + HBr
KOH + HBr → KBr + H2O
C2H5CONHBr + KOH → C2H5NCO + KBr + H2O KOH-
C2 H5NCO + 2KOH → C2H5NH2+K2CO3
C2 H5 CONH2 + Br2 + 4KOH → C2H5NH2 + 2KBr + K2CO3 + 2H2O
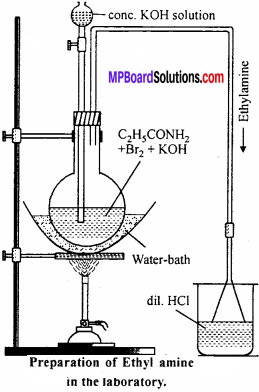
Procedure:
In a round bottom distillation flask equivalent quantities of bromine and propanamide is taken and 10% KOH solution is added in it. Now 50% KOH solution is added in excess and the flask is heated on water both up to 57-67°C. When the solution becomes colourless, ethyl amine starts to be distilled which is absorbed in dil. HCl.
Physical properties:
It is a colourless liquid with an odour like ammonia which is soluble in water and organic solvent. It is a combustible substance. Its boiling point is 19°C.
![]()
Question 9.
Write laboratory method of preparation of aniline. Give all chemical equations of this process.
Or
Describe commercial method of preparation of aniline.
Answer:
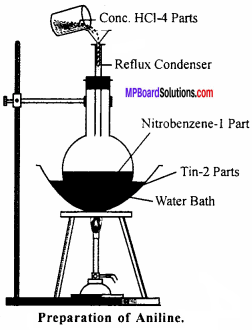
Laboratory Method for the Preparation of Aniline:
Aniline is prepared in the laboratory by the reduction of nitro-benzene with tin and hydrochloric acid.
C6H5NO2 +6[H] C6H5NH2 + 2H2O
Experiment:
Nitro-benzene (20 g) and granulated tin (40 g) are taken in a round-bottom flask and a reflux condenser is fitted. Now hydrochloric acid (100 ml) is added in small amounts (10 ml at a time). The flask is shaken after each addition of acid and the temperature is not allowed to rise above 90° C. The flask is heated on a water bath till the smell of nitro-benzene disappears. On cooling the flask, a solid mass of molecular formula (C6H5 NH2 .HCl)2 .SnCl4 separates out.
Sn + 4HCI → SnCI4 + 4H
C6H5NO2 + 6[H] ) → C6H5NH2 + 2H2O
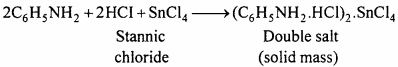
The solid mass is treated with concentrated NaOH solution. So that a clear alkaline solution is obtained. Aniline is liberated and floats as a dark brown coloured oil.
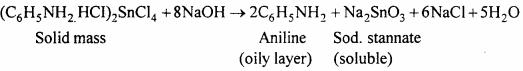
From this aniline is obtained by steam distillation.
Commercially aniline is manufactured by reduction of nitrobenzene. This is done in presence of Fe water and cone. HCl.
Fe + 2HCl → FeCl2 + 2H
C6H5 NO2 + 6H → C6H5NH2 + 2H2O
Question 10.
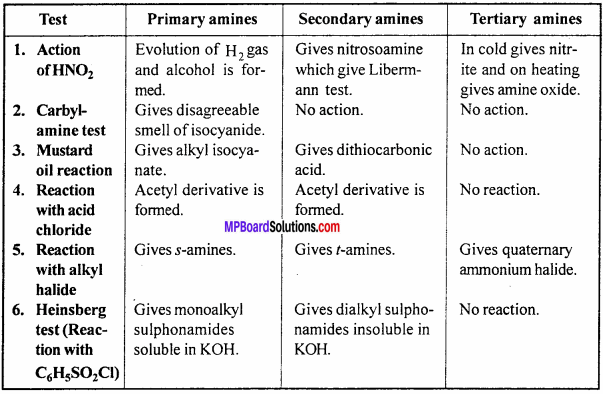
Distinguish among p, s and t amines under the following points:
- Reaction with HNO2
- Carbyl amine reaction
- Mustard oil reaction
- Reaction with acid halide
- Reaction with alkyl halide
- Reaction with C6H5SO2Cl.
Answer:
Question 11.
Write down the chemical equation representing preparation of nitrobenzene in laboratory and give the following chemical reaction of nitrobenzene:
(a) Nitration
(b) Sulphonation.
Answer:
Preparation of nitrobenzene in laboratory: Nitrobenzene is prepared in laboratory by treating benzene with a mixture of cone. HNO3 and cone. H2SO4 at 60°C.
![]()
Reaction of nitrobenzene:
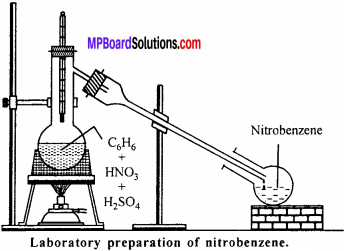
(a) Nitration:
Nitrobenzene forms m – dinitrobenzene when heated with fuming HNO3 and cone. H2SO4 .
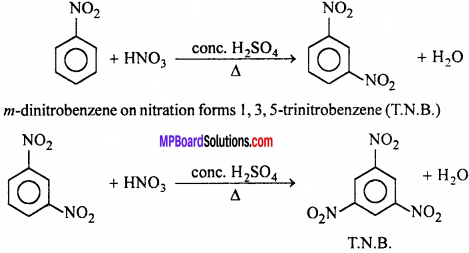
(b) Sulphonation:
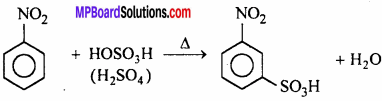
Nitrobenzene forms m -nitrobenzene sulphonic acid on heating with fuming H2SO4 .
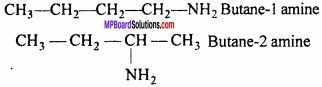
Question 12.
Write the possible isomers of C4H11N.
Answer:
(i) Position isomerism:
(ii) Chain isomerism:

(iii) Metamerism:
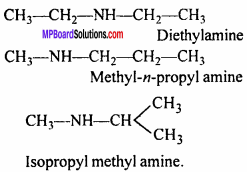
Question 13.
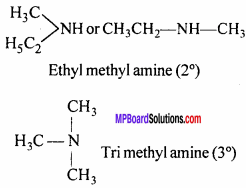
Write the type of possible isomers of C3H9N.
Answer:
(i) Functional group isomerism:
CH3—CH2—CH2—NH2
n-propyl amine (1°)
All functional group are isomers.
(ii) Position Isomerism:
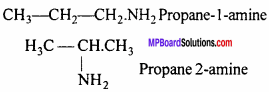
All are position isomers.