In this article, we will share MP Board Class 11th Chemistry Solutions Chapter 8 Redox Reactions Pdf, These solutions are solved by subject experts from the latest edition books.
MP Board Class 11th Chemistry Solutions Chapter 8 Redox Reactions
MP Board Class 11 Chemistry Redox Reactions Textbook Questions and Answers
Question 1.
Assign oxidation number to the underlined elements in each of the following species:
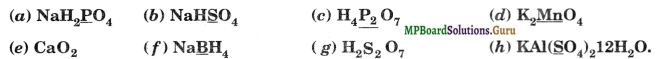
Solution.
(a) Let the oxidation number of P be x. The oxidation number of each atom is shown above its symbol,
![]()
Sum of oxidation numbers of various atoms in NaH2PO4 is zero,
hence 1(+ 1) + 2(+ 1) + 1(x) + 4(- 2) = 0 or x – 5 = 0 or x = + 5
Thus, the oxidation number of P in NaH2PO4 = + 5.
(b) In the similar way as discussed, the sum of oxidation numbers in ![]() will be,
will be,
1(+ 1) + 1(+ 1) + x + 4(- 2) = 0 or x = + 6 Thus, the oxidation number of S in NaH2PO4 = + 6.
+ 1 x -2
(c) The sum of oxidation numbers in![]() , will be
, will be
4(+ 1) + 2(x) + 7(- 2) = 0 or x = + 5 Thus, the oxidation number of P in H4P2O7 = + 5.
(d) The sum of oxidation numbers in ![]() , will be
, will be
2(+ 1) + l(x) + 4(- 2) = 0 or x = + 6.
Thus, the oxidation number of Mn in K2MnO4 = + 6.
(e) The oxidation number of Ca is + 2. Thus, the sum of oxidation numbers in ![]() will be
will be
+2+ 2 x = 0 or x = -1 or x = – 1 Thus, oxidation number of oxygen in CaO2 = – 1.
(f) In NaBH4, H is present as hydride ion. Therefore, its oxidation number is – 1. Thus,
for , the sum of oxidation numbers is 1(+ 1) + x + 4(- 1) = 0 or x = + 3
Thus, the oxidation number of B in NaBH4 = + 3.
(g) The sum of oxidation numbers in, ![]() will be given as
will be given as
2(+ 1) + 2(x) + 7(- 2) = 0 or x = + 6
Thus, the oxidation number of S in Na2S2O7 = + 6.
(h) The sum of oxidation numbers in,
![]()
+ 1 + 3 + 2x + 8(- 2) + 12(2 x 1 – 2) = 0 or x = + 6
Alternatively, since H2O is a neutral molecule, therefore, sum of oxidation numbers of all the atoms in H20 may be taken as zero. As such water molecules can be ignored while computing the oxidation number of S. Accordingly,
+ l + 3 + 2x-16 = 0 or x = + 6 Thus, the oxidation number of S in KAl(SO4)2.12 H2O = + 6.
Question 2.
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalize your results?
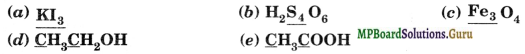
Solution.
(a) In KI3, since the oxidation number of K is + 1, therefore, the average oxidation number of iodine = – 1/3. But the oxidation number cannot be fractional. Therefore, in such cases we must consider its structure, (![]() ). InI3– ion, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine
). InI3– ion, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine
atoms forming the I2 molecule is zero while that of iodine forming the coordinate bond is -1.
(b) Conventional method. The average oxidation number in ![]() is calculated as:
is calculated as:
2(+ 1) + 4x + 6(- 2) = 0 or x = + 2.5.
But it is wrong because all the four S atoms cannot be in the same oxidation state, in the compound.
Thus, in such cases the oxidation number is calculated by chemical bonding method, i.e., the oxidation number is computed on the basis of its structure. The structure of H2S4O6 is as follows:
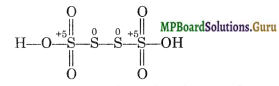
The O.N. of each of the S-atoms linked with each other in the middle is zero while that of the remaining two S-atoms is + 5.
(c) Conventional method. The average oxidation number of Fe in ![]() will be 3x + 4(- 2) = 0
will be 3x + 4(- 2) = 0
or x = 8/3.
Fe3O4 is a mixed oxide therefore its oxidation number is calculated by Stoichiometry method. Accordingly, Fe3O4 shall be written as: ![]()
Fe has O.N. of + 2 and + 3.
(d) Conventional method. The average oxidation number of carbon in C2H5OH or
![]() will be
will be
2x + 6(+ 1) + 1(- 2) = 0 or x = – 2.
On the basis of chemical bonding method as carbon C-2 is attached to three H-atoms (less electronegative than carbon) img and one CH2OH group (more electrone- gative than carbon), therefore, O.N. of C-2 will be = 3(+ 1) + x + 1(- 1) = 0 or x = – 2. The carbon, C-l is, however, attached to one OH group (O.N. – 1) and one CH3 group (O.N. = + 1), therefore, O.N. of C-l = + 1 + 2(+ 1) + x + 1(- 1) = 0 or x = – 2.
(e) Conventional method. The oxidation number of carbon in CH3COOH or img will be
2x + 4 – 4 = 0 or x = 0.
Chemical bonding method. The carbon C-2 is attached to three H-atoms (less electronegative than carbon) img and one -COOH group (more electronegative than carbon), therefore, O.N. of C-2 = 3(+ 1) + a: + 1(- 1) = 0 or x = -2
The carbon, C-l is, however, attached to one oxygen atom by a double bond, one OH group (O.N. = – 1) and one CH3 group (O.N. = + 1), therefore, O.N. of C-l = + 1 + x + 1(- 2)+ 1(— 1) = 0 or x + 2.
Question 3.
Try all possible approaches to justify that the following reactions are redox reactions.
(a) CuO(s) + H2(g) → Cu(s) + H2O(g)
(b) Fe2O3 (s) + 3CO(g) → 2Fe(s) + 3CO2(g)
(c) 4BCl3(g) + 3LiAlH4(s) → 2B2H6(g) + 3LiCl(s) + 3A1Cl3(S)
(d) 2K(s) + F2(g) → 2K+F–(s)
(e) 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g).
Solution.
(a) The chemical equation with oxidation number of each element shown on its symbol is written as:
![]()
Here, O is removed from CuO, therefore, it is reduced to Cu while O is added to H2 to form H2O2, therefore hydrogen is oxidised. Further, O.N. of Cu decreases from + 2 in CuO to 0 in Cu but that of H increases from 0 in H2 to + 1 in H20. Therefore, CuO is . reduced to Cu but H2 is oxidised to H2O. Thus, this is a redox reaction.
(b) The chemical equation of the reaction is:
![]()
Here O.N. of Fe decreases from + 3 in Fe2O3 to 0 in Fe while that of C increases from + 2 in CO to + 4 in CO2. Further, oxygen is removed from Fe2O3 and added to CO, therefore, Fe2O3 is reduced while CO is oxidised. Thus, this is a redox reaction.
(c) The chemical equation along with oxidation number of each element is:
![]()
Here, O.N. of Br decreases from + 3 in BrCl3 to – 3 in B2H6 while that of H increases from – 1 in LiAlH4 to + 1 in B2H6. Therefore, BCl3 is reduced while LiAlH4 is oxidised. Further, H is added to BrCl3 but is removed from LiAlH4, therefore, BCl3 is reduced while LiAlH4 is oxidised. Thus, it is a redox reaction.
(d) The chemical equation along with oxidation number of each element will be written as:
![]()
Each K atom has lost one electron to form K+ while F2 has gained two electrons to form two F– ions. Therefore, K is oxidised while F2 is reduced. Thus, it is redox reaction.
(e) In the equation,
![]()
O.N. of N increases from -3 in NH3 to + 2 in NO while that of O decreases from O in O2 to-2 inNO or H2O.
Therefore, NH3 is oxidised while O2 is reduced.
Further H has been removed from NH3 but added to O2. Therefore, NH3 has been oxidised while O2 is reduced. Thus, this is a redox reaction.
![]()
Question 4.
Fluorine reacts with ice and results in the change:
![]()
Justify that this reaction is a redox reaction. If oxygen of HOF disproportionates at room temperature, then what reaction is possible? Solution.
In the given reaction O.N. of F2 changes from zero to – 1 in HF and HOF whereas O.N. of oxygen changes from – 2 in H2O to zero in HOF. Thus, F2 is reduced, whereas oxygen is oxidised and, therefore, it is a redox reaction.
HOF is a highly unstable molecule and hence decomposes to form O2 and HF
![]()
If oxygen of HOF disproportionates, then in the disproportionation reaction oxygen must have three oxidation states. In simple words, the oxidation state of oxygen in one of the products should be lower than O in HOF whereas in the other product it should be higher. The oxidation state of oxygen is zero in HOF. It decrease its oxidation state to – 2 if HOF gets reduced to H2O and also it can increase its oxidation state to + 2 if HOF gets oxidised to OF2. Therefore, the possible reaction is:
![]()
Question 5.
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, Cr2O72- and NO3–. Suggest structures of these three compounds. Count for the fallacy.
Solution.
(i) Oxidation number of S in H2SO5 On the basis of conventional method, the oxidation number of S in H2SO5 comes out to be + 8 as shown below.
2 (+ 1) + x + 5(- 2) = 0 or x = + 8
This is impossible because the maximum oxidation number of S cannot be more than six since it has only six electrons in the valence shell. This fallacy is overcome by calcu¬lating the O.N. of S by chemical bonding method.
The structure of H2SO5 along with oxidation number of different atoms is:
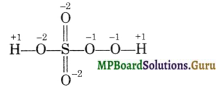
Hence, 2(+ 1) + 3(- 2) + x + 2(- 1) = 0 or x = + 6
(ii) Oxidation number of Cr in Cr2O72- According to conventional method, oxidation number of Cr is: x + 5 (- 2) = 0 or x = + 10. This is impossible because the maximum oxidation number of Cr cannot be more than six since it has a maximum of six electrons (3d54s2) in the outer orbital configuration which can participate in bonding. This fallacy is removed by calculating oxidation number of Cr by chemical bonding method. The structure of CrO5 is:
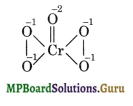
From the structure, the oxidation number of Cr can be calculated as follows: x + 4(- 1) + 1(- 2) = 0 or x = + 6
It should be noted that four O atoms have peroxy linkage (oxidation number = – 1) and O held by double bond has oxidation number = – 2.
(iii) Oxidation number of N in NO3–
According to conventional method, oxidation number of N in NO3– = x + 3(- 2) = — 1 or x = + 5
According to chemical bonding method, the structure of nitrate ion is:
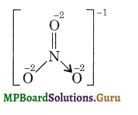
Hence the oxidation number of nitrogen is,
x + 3(- 2) = – 1 or x = + 5
Thus, the oxidation number of N in NO3– whether one calculates by conventional method or by chemical bonding method is same.
Question 6.
Write formulas for the following compounds:
(a) Mercury (II) chloride
(b) Nickel (II) sulphate
(d) Thallium (I) sulphate
(e) Iron (III) Sulphate
(f) Chromium (III) oxide.
Solution:
(a) Hg(II)Cl2
(b) Ni(II)SO4
(c) Sn(IV)O2
(d) Th2(I)SO4
(e) Fe2(III)(SO4)3
(f) Cr2(III)O3
Question 7.
Suggest a list of substances where carbon can exhibit oxidation states from – 4 to + 4 and nitrogen from – 3 to + 5.
Solution.
Various compounds of carbon and nitrogen formed in different oxidation states are tabulated below:
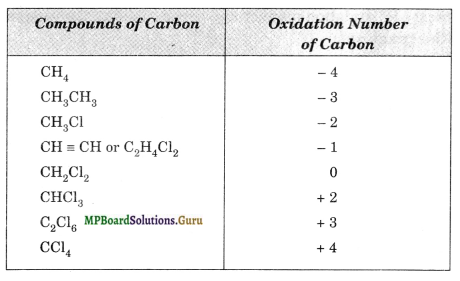
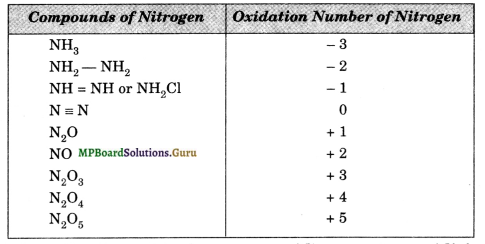
Question 8.
While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
Solution.
Any substance can act as an oxidising or reducing agent if one of the elements in the given compound is present in its intermediate oxidation state. Thus, during the reaction it can either increase its oxidation number (reducing agent) or decrease its oxidation number (oxidising agent).
(i) In SO2, oxidation number of S is + 4. In principle, S can have a minimum oxidation number of – 2 and maximum of + 6. Therefore, S in SO2 can either decrease or increase its oxidation number and hence can act both as an oxidising as well as a reducing agent.
(ii) In H2O2, the oxidation number of O is – 1. In principle, O can have a minimum oxidation number of- 2 and maximum of zero (+ 2 is possible only with 0F2). Therefore, O in H2O2 can either decrease its oxidation number from – 1 to – 2 or can increase its oxidation number from – 1 to zero. Therefore, H2O2 acts both as an oxidising as well as a reducing agent.
(iii) In O3, the oxidation number of O is zero. It can only decrease its oxidation number from zero to -1 or – 2, but cannot increase to + 2. Therefore, O3 acts only as an oxidant.
(iv) In HNO3, nitrogen atom is in its maximum oxidation state of + 5. Therefore, it can only decrease its oxidation number and hence can act as an oxidant only.
Question 9.
Consider the reactions:
(а) 6CO2 (g) + 6H2O(l) → C6H12O6(s) + 6O2(g)
(b) O3(g) + H2O2(l) → H2O (l) + 2O2(g)
Why it is more appropriate to write these reactions as;
(a) 6CO2 (g) + 12H2O(l) → C6H12O6(s) + 6 H2O(l) + 6O2(g)
(b) O3(g) + H2O2(l) → H2O (l) + O2(g) + O2(g)
Also suggest a technique to investigate the paths of above (a) and (b) redox reactions.
Solution.
(a) The given reaction occurs during photosynthesis. Although the mechanism of photosynthesis is very complex but the process/reaction may be visualized to occur in two steps.
(i) In the first step, H2O decomposes to give H2 and O2 in presence of chlorophyll.
(ii) In step 2, the H2 thus produced reduces CO2to C6H12O6 along with the formation of some H2O molecules as shown on next page.
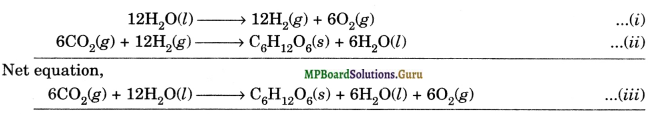
It is more appropriate to write the equation for photosynthesis as equation (iii) because the equation clearly illustrates that 12H2Omolecules are used per molecule of carbohydrate formed and 6H2O molecules are produced during the process.
(b) In equation (b) dioxygen gas is written twice. The purpose of writing O2 two times suggests that O2 is obtained from each of the two reactants as shown by equations given below:
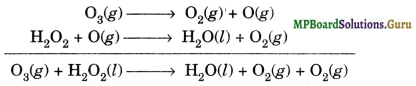
The path of reactions (a) and (b) can be determined by tracer techniques i.e., by using different isotopes of elements. For example, using H2O18 in reaction (a) or by using H2O18 or O318 in reaction (b).
Question 10.
The compound AgF2 is unstable. However, if formed, the compound acts as a very strong oxidising agent. Why?
Solution.
In AgF2, oxidation state of Ag is + 2 which is very very unstable. Since Ag can exist in a stable state of + 1, therefore, it quickly accepts an electron to form the more stable + 1 oxidation state.
Ag2+ + e–→ Ag+
Therefore, AgF2, if formed, will act as a strong oxidising agent.
Question 11.
Whenever a reaction between an oxidising agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if oxidising agent is in excess. Justify this statement giving three illustrations.
Solution.
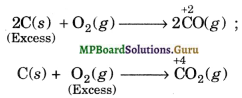
(i) Illustration 1. Let us consider reaction between C (reducing agent) and O2 (oxidising agent). If excess of carbon is burnt in a limited supply of O2, CO is formed in which the oxidation state of C is + 2. If, however, excess of O2 is used, the initially formed CO gets oxidised to C02 in which the oxidation state of C is + 4.
(ii) Illustration 2. Reaction between P4 (reducing agent) and Cl2 (oxidising agent). When excess of P4 is used, PCl3 is formed in which the oxidation state of P is + 3. If, how¬ever, excess of Cl2 is used, the initially formed PCl3 reacts further to form PCl5 in which the oxidation state of P is + 5
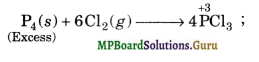
![]()
(iii) Illustration 3. Reaction between Na (reducing agent) and O2 (oxidising agent). When excess of Na is used, sodium oxide is formed in which the oxidation state of O is – 2. If, however, excess of O2 is used, Na2O2 is formed in which the oxidation state of O is – 1 which is higher than – 2.
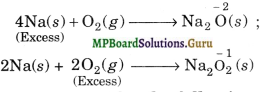
Question 12.
How do you account for the following observations:
(а) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction.
(b) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get pungent-smelling gas HCl, but if the mixture contains bromide then we get red vapour of bromine. Why?
Solution.
(a) Toluene can be oxidised to benzoic acid in acidic, basic and neutral medium as per the following redox reactions
(i) Acidic medium
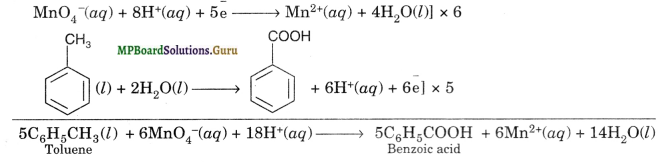
(ii) In Basic and neutral medium
![]()
(b) When cone. H2SO4 is added to an inorganic mixture containing chloride, a pungent smelling gas, HCl, is produced because a stronger acid can displace a weaker acid from its salt.
2NaCl + 2H2SO4 → 2NaHSO4 + 2HCl
Since HCl is a very weak reducing agent, it cannot reduce H2SO4 to SO2 and hence HCl is not oxidised to Cl2 i.e., the following reaction will not be possible.
2HCl + H2SO4 → Cl2 + SO2 + 2H2O
However, when the mixture contains bromide ion, the initially produced HBr being a stronger reducing agent reduces H2SO4 to SO2 and is itself oxidised to produce red vapour of Br2.
2NaBr + 2H2SO4 → 2NaHSO4 + 2HBr
2HBr + H2SO4 → Br2 + SO2 + 2H2O
Question 13.
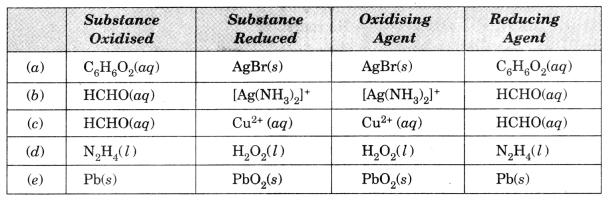
Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions.
(a) 2AgBr (s) + C6H6O2(aq) → 2Ag(s) + 2HBr(aq) + C6H4O2(aq)
(b) HCHO(l) + 2[Ag(NH3)2]+(aq) + 3OH–(aq) →
2Ag(s) + HCOO– (aq) + 4NH3(aq) + 2H2O(l)
(c) HCHO(l) + 2Cu2+ (aq) + 5OH–(aq) → Cu2O(s) + HCOO–(aq) + 3H2O(l)
(d) N2H4(l) + 2H2O2(l) → N2(g) + 4H2O(l)
(e) Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
Solution.
The list of substance oxidised, reduced, etc., for the given reactions are tabulated below :
Question 14.
Consider the reactions:
2S2O32-(aq) + I2(s) → S4O62-(aq) + 2I–(aq)
S2O32- (aq) + 2Br2(l) + 5H2O(l) → 2SO42-(aq) + 4Br–(aq) + 10H+(aq)
Why does the same reductant, thiosulphate react differently with iodine and bromine?
Solution.
The average O.N. of S in S2O32- is + 2 while in S4O62- it is + 2.5. The O.N. of S in SO42- is + 6. Since Br2 is a stronger oxidising agent that I2, it oxidises S of S2O32- to a higher oxidation state of + 6 and hence forms SO42- ion. I2, however, being a weaker oxidising agent oxidises S of S2O32- ion to a lower oxidation of + 2.5 in S4O62-ion. It is because of this reason that thiosulphate reacts differently with Br2 and I2.
![]()
Question 15.
Justify giving reactions that among halogens, fluorine is the best oxidant and among Hydrohalic compounds, hydriodic acid is the best reductant.
Solution. The oxidising power of halogens decreases in the order: F2 > Cl2 > Br2 > I2. This is evident from the observation that F2 oxidises Cl– to Cl2, Br– to Br2,I– to I2 ; Cl2 oxidises Br– to Br2 and I– to I2 but not F to F2.Br2, however, oxidises I– to I2 but not F– to F2, and Cl– to Cl2.
F2(g) + 2Cl–(aq) → 2F–(aq) + Cl2(g);
F2(g) + 2Br–(aq) → 2F–(aq) + Br2(l)
F2(g) + 2I –(aq) → 2F–(aq) + I2(s);
Cl2(g) + 2Br– (aq) → 2Cl– (aq) + Br2(l)
Cl2(g) + 2I–(aq) → 2Cl–(aq) + I2(s)
and Br2( l) + 2I– → 2Br–(ag) + I2(s)
Thus, F2 is the best oxidant.
Among hydrohalic acids, the reducing power decreases in the order: HI > HBr > HC1 > HF. Thus, HI and HBr reduce H2SO4 to SO2 while HCl and HF do not.
2HBr + H2SO4 → Br2 + SO2 + 2H2O
2HI + H2SO4 → I2 + S02 + 2H20
Further I– reduces Cu2+ to Cu+ but Br– does not.
2Cu2+(aq) + 4I–(aq) → Cu2I2(s) + I2(aq)
Cu2+(aq) + 2Br– → No reaction.
Further among HCl and HF, HCl is a stronger reducing agent than HF because HCl reduces MnO2 to Mn2+ but HF does not.
MnO2(s) + 4HCl(aq) → MnCl2(aq) + Cl2(g) + 2H2O
MnO2(s) + 4HF(l) → No reaction.
Thus, the reducing character of hydrohalic acids decreases in the order: HI > HBr > HCI > HF.
Question 16.
Why does the following reaction occur?
XeO64-(aq) + 2F–(aq) + 6H+(aq) → XeO3 (s) + F2(g) + 3H2O(l)
What conclusion about the compound Na4XeO6 (of which XeO64- is a part) can be drawn from the reaction?
Solution.
The balanced equation along with O.N. of the elements above their symbols will be as:
![]()
In the equation the, O.N. of Xe decreases from + 8 in XeO64- to + 6 in XeO3 while that of F increases from – 1 in F– to 0 in F2. Therefore, XeO64- is reduced while F~ is oxidised. This reaction occurs because Na2XeO64- (or XeO64-) is stronger oxidising agent than F2.
Question 17.
Consider the reactions:
(а) H3PO2(aq) + 4AgNO3(aq) + 2H2O(l) → H3PO4(aq) + 4Ag(s) + 4HNO3(aq)
(b) H3PO2(aq) + 2CuSO4(aq) + 2H2O(l) → H3PO4(aq) + 2Cu(s) + H2SO4(aq)
(c) C6H5CHO(l) + 2[Ag(NH3)2]+(aq) + 3OH–(aq) →
C6H5CHO(aq) + 2Ag(s) + 4NH3(aq) + 2H2O(l)
(d) C6H5CHO(l) + 2Cu2+(aq) + 5OH (aq) → No change observed.
What inference do you draw about the behaviour of Ag+ and Cu2+ from these reactions?
Solution.
Reactions (a) and (b) indicate that H3PO2 (hypophosphorous acid) is a reducing agent and thus reduces both AgNO3 and CuSO4 to Ag and Cu respectively. Conversely, both AgNO3 and CuSO4 act as oxidising agent and thus oxidise H3PO2 to H3PO4. Reaction (c) suggests that [Ag(NH3)2]+ oxidises C6H5CHO (benzaldehyde) to C6H5COO– (benzoate ion) but reaction (d) indicates that Cu2+ ions cannot oxidise C6H5CHO to C6H5COO–. Therefore, from the above reactions, we conclude that Ag+ ion is a stronger oxidising agent than Cu2+ ion.
Question 18.
Balance the following redox reactions by ion-electron method.
(а) MnO4–(aq) + I–(aq) → MnO2(s) + I2(s) (in basic medium)
(b) MnO4– (aq) + SO2(g) → Mn2+(aq) + HSO4– (in acidic solution)
(c) H2O2(aq) + Fe2+(aq) → Fe3+(aq) + H2O(l) (in acidic solution)
(d) Cr2O72-(aq) + SO2(g) → Cr3+(aq) + SO42-(aq) (in acidic solution)
Solution.
(a) Step 1. The Skeletal equation of the reaction is:
![]()
Step 2. Writing the two half-reactions:
Oxidation half reaction, I–(aq) → ![]()
![]()
Step 3. Balancing of atoms in the half equation 2I–(aq) → I2(s)
In the other equation the step is not required because Mn is balanced.
Step 4. Balancing of charges in the half-reactions by adding electrons
2I–(ag) → I2(s) + 2e–
![]()
Step 5. Balancing of oxygen atoms and H atoms
MnO4– (aq) + 3e– → MnO2(s) + 2H2O(l)
To balance H atoms, we add 4H+ ions on the left
MnO4–+ 4H+(aq) → MnO2(s) + 2H2O(l)
As the reaction is taking place in basic solution, therefore for the 4H+ ions we add 4 OH– ions to both sides of the equation.
MnO4–(aq) + 4H+(aq) + 4OH–(aq) → MnO2(s) + 2H2O(l) + 4OH–(aq)
As H+ and OH– ions form water, therefore the resultant equation is
MnO4–(aq) + 2H2O(l) + 3e– → MnO2(s) + 4OH–(aq)
Step 6.
(a) Balancing of electrons in the two half equations
2I–(aq) → I2(s) + 2e– ………………………………………..(i)
MnO4–(aq) + 2H2O(l) + 3e– → MnO2(s) + 4OH–(aq) …………………………………………(ii)
Multiply (i) with 3 and (ii) with 2 and add
6I–(aq) + 2MnO4–(aq) + 4H2O(l) → 3I2(s) + 2MnO2(s) + 8OH–(aq)
(b) By applying the procedure as discussed in the equations above Oxidation half equation will be:
SO2(g) + 2H2O(l) →HSO4–(aq) + 3H+ (aq) + 2e– ……………………………….(i)
The reduction half equation will be:
MnO4–(aq) + 8H+(ag) + 5e–→ Mn2+(aq) + 4H2O(l) ……………………………………………………..(ii)
Multiply equation (i) by 5 and equation (ii) by 2 and adding the equations, we get,
2MnO4–(aq) + 5SO2(g) + 2H2O(l) + H+(aq) → 2Mn2+ (aq) + 5HSO4–(aq)
(c) By applying the first 5 steps as discussed above, the oxidation half equation will be:
Fe2+(aq) → Fe3+(aq) + e– ……………..(i)
The reduction half equation will be:
H2O2(aq) + 2H+(ag) + 2e– → 2H2O(l) …………………(ii)
Multiply equation (i) by 2 and adding it to equation (ii) we get
H2O2(aq) + 2Fe2+ (aq) + 2H+ (aq) → 2Fe3+ (aq) + 2H2O(l)
(d) Following the procedure discussed in details and applying 5 steps the balanced half-reaction equations are:
Oxidation half equation:
SO2(g) + 2H2O(l) → SO42-(aq) + 4H+(aq) + 2e– ……………………(i)
Reduction half equation:
Cr2O72-(ag) + 14H+(aq) + 6e– → 2Cr3+ (aq) + 7H2O(l) …………………………………………………(ii)
Multiplying equation (i) by 3 and adding it to equation (ii), we get
Cr2O72-(aq) + 3SO2(g)+2H+(aq) → 2Cr3+ (aq) + 3SO42- (aq) + H2O(l).
![]()
Question 19.
Balance the following equations in basic medium by ion-electron method and oxidation number method and identify the oxidising agent and the reducing agent.
(а) P4(s) + OH– (aq) → PH3(g) + HPO2–(aq)
(b) N2H4(l) + CIO3–(aq) → NO(g) + Cl–(aq)
(c) Cl2O7(g) + H2O2(aq) → CIO2–(aq) + O2(g) + H+
Solution.

P4 acts both as an oxidising as well as reducing agent.
Oxidation number method
Total decrease in O.N. of P4 in PH3 = 3 × 4 = 12
Total increase in O.N. of P4 in H2PO2– =1×4 = 4
(i) To balance increase/decrease in O.N. multiply PH3 by 1 and H2PO2– by 3. After doing so we get the equation,
P4(s) + OH–(ag) → PH3(g) + 3H2PO2–(aq)
(ii) The oxygen atoms are balanced by multiplying OH– by 6 and we get the equation,
P4(s) + 6OH–(aq) → PH3(g) + 3H2PO2–(aq)
(iii) To balance H atoms, 3H2O are added to L.H.S. and 3 OH– to the R.H.S. Thus, the equation is,
P4(s) + 6OH–(aq) + 3H2O(l) → PH3(g) + 3H2PO2–(aq) + 3OH–(ag)
or The correct balanced equation is:
P4(s) + 3OH–(aq) + 3H2O(l) → PH3(g) + 3H2PO2–(ag) ………………………………. (i)
Ion electron method. The two half-reactions are:
Oxidation half-reaction:
P4(s) → H2PO2– …………………………… (ii)
Balancing P atoms, we have,
![]()
Balance O.N. by adding electrons,
P4(s) → 4H2PO2–(ag) + 4e–
Balance charge by adding OH- ions
P4(s)+ 8OH–(ag) → 4H2PO2–(ag) + 4e– ………………… (iii)
O and H get automatically balanced. Thus, equation (iii) represents the balanced oxidation half reaction.
Reduction half reaction:
![]()
Balancing P atoms, we have,
P4(s) →4PH3 (g)
Balance O.N. by adding electrons,
P4(s) + 12e–→ 4PH3(g)
Balance charge by adding OH- ions,
P4(s) + 12e– →4PH3(g) + 12OH–(ag)
Balance O atoms by adding 12H2O to L.H.S. of above equation.
P4(s) + 12H2O(l) + 12e– → 4PH3(g) + 12OH–(ag) …………………………………………… (v)
To cancel out electrons, multiply equation (iii) by 3 and add it to equation (v), we have,
4P4(s) + 24OH–(ag) + 12H2O(l) → 4PH3(aq)
+ 12H2PO2–(aq) + 12 H2O(l) + 12OH–(ag)
or P4(g) + 3 OH–(aq) + 3H2O(l) → PH3(ag) + 12H2PO2–(aq) ……………………………………………………….. (vi)
Thus, equation (vi) represents the correct balanced equation.
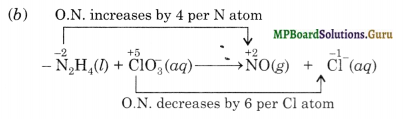
Therefore, N2H4 acts as the reducing agent while CIO3– acts as the oxidising agent.
Oxidation number method
Total increase in oxidation number of N = 2×4 = 8
Total decreases in oxidation number of Cl = 1 × 6 = 6
Therefore, to balance increase/decrease in oxidation number multiply N2H4 by 3 anc CIO3–by 4, we have,
3N2H4(l) + 4CIO3–(ag) → NO(g) + Cl–(aq)
To balance N and Cl atoms, multiply NO by 6 and Cl– by 4, we have,
3N2H4(l) + 4CIO3–(aq) → 6NO(g) + 4Cl–( aq)
Balance O atoms by adding 6H2O,
3N2H4(l) + 4CIO3– (aq) → 6NO(g) + 4Cl–( aq) + 6H2O(l) …………………………………(i)
H atoms get automatically balanced and thus equation (Z) represents the correct balanced equation.
Ion electron method. Two half-reactions are:
Oxidation half equation:
![]()
Balance N atoms,
N2H4(l) → 2NO(g)
Balance O.N. by adding electrons,
N2H4(l) → 2NO(g) + 8e–
Balance charge by adding OH- ions,
N2H4(l) + 8OH–(aq) → 2NO(g) + 8e–
Balance O atoms by adding 6H2O,
N2H4(l) + 8OH–(aq) → 2NO(g) + 6H2O(l) + 8e– ………………………………………(iii)
Thus, equation (ii) represents the correct balanced oxidation half equation.
Reduction half equation:

Balance oxidation number by adding electrons,
CIO3– (aq)+ 6e– → Cl–(aq)
Balance charge by adding OH ions,
CIO3– (aq) + 6e–→ Cl–(aq) + 6OH–(aq)
Balance O atoms by adding 3H2O,
CIO3– (aq) + 3H2O(l) + 6e–→ Cl–(aq)+ 6OH–(aq) ………………………………….. (iii)
Thus, equation (iii) represents the correct balanced reduction half equation.
To cancel out electrons gained and lost, multiply equation (ii) by 3 and equation (iii) by 4 and add, we have,
3N2H4(l)+ 4CIO3–(aq) → 6NO(g) + 4Cl–(aq) + 6H2O(l)
This represents the correct balanced equation.
Thus, Cl2O7(g) acts an oxidising agent while H2O2(aq) as the reducing agent. Oxidation number method
Total decrease in oxidation number of Cl2O7 = 4× 2 = 8 Total increase in oxidation number of H2O2 = 2 × 1 = 2
∴ To balance increase/decrease in O.N. multiply H2O2 and O2 by 4, we have,
Cl2O7(g) + 4H2O2(aq) → CIO2–(aq) + 4O2(g)
To balance Cl atoms, multiply CIO2– by 2, we have,
Cl2O7(g) + 4H2O2(aq) → 2CIO2–(aq) + 4O2(g)
To balance O atoms, add 3H2O to R.H.S., we have,
Cl2O7(g) + 4H2O2(aq) → 2CIO2–(aq) + 4O2(g) + 3H2O(l)
To balance H atoms, add 2H2O to R.H.S., and 2OH– to L.H.S., we have,
Cl2O7(g) + 4H2O2(aq) + 2OH–(g) → 2CIO2–(aq) + 4O2(g) + 5H2O
This represents the balanced redox equation.
Ion electron method. Two half-reactions are:
Oxidation half equation:
![]()
Balance O.N. by adding electrons,
H2O2(aq) → O2(g) + 2e–
Balance charge by adding 0H~ ions,
H2O2(aq) + 2OH–(aq) → O2(g) + 2e–
Balance O atoms by adding H2O,
H2O2(aq) + 2OH–(aq) → O2(g) + 2H2O(l) + 2e– ……………………………… (i)
Reduction half equation:
![]()
Balance Cl atoms;
Cl2O7(g) → 2CIO2–(aq)
Balance oxidation number by adding electrons,
2CIO2–(aq) + 8e– → 2CIO2–(aq)
Add OH ions to balance charge,
Cl2O7(g) + 8e– → 2CIO2–(aq) + 6OH–
Balance O atoms by adding 3H20 to L.H.S., we have,
Cl2O7(g) + 3H2O(l) + 8e– → 2CIO2–(aq) + 6OH–(aq) …………………………………………….. (ii)
To cancel out electrons, multiply equation (t) by 4 and add it to equation (it), we have,
4H2O2(aq) + 8OH–(aq) + Cl2O7(g) + 3H2O(l) →
2CIO2–(aq) + 6OH–(aq) + 4O2(g) + 8H2O(l)
or Cl2O7(g) + 42O2(aq) + 2HO–(aq) → 2CIO2–(aq) + 4O2(g) + 5H2O(l).
Question 20.
Write four informations about the reaction:
(CN)2(g) + 2OH–(aq) → CN–(aq) + CNO (aq) + H2O(l)
Solution.
- It is a disproportionate reaction.
- Cyanogen (CN)2 gets simultaneously reduced to CN– ion as well as oxidised to cyanate ion.
- Oxidation number of N in (CN)2 is – 3 while that in CN– is – 2 and in CNO– is – 5.
- The reaction occurs in a basic medium.
Question 21.
The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write a balanced ionic equation for the reaction.
Solution.
The skeletal equation is:
Mn3+ (aq) → Mn2+(aq) + MnO2 (s) + H+(aq)
Oxidation half equation:
![]()
Balance oxidation number by adding electrons,
Mn3+ (aq) → MnO2 (s) + e–
Balance charge by adding H+ ions,
![]()
Balance O atoms by adding H2O,
![]()
Reduction half equation:
Mn3+ (aq) → Mn2+
Balance oxidation number by adding electron,
Mn3+ (aq)+ e– → Mn2+(aq) ……………………………………………… (ii)
Adding equation (i) and equation (ii), the balanced equation for the disproportionation reaction is
2Mn3+ (aq) + 2H2O(l) → MnO2 (s) + Mn2+(aq) + 4H+(aq).
Question 22.
Consider the elements: Cs, Ne, I, F
(a) Identify the element that exhibits -ve oxidation state.
(b) Identify the element that exhibits +ve oxidation state.
(c) Identify the element that exhibits both +ve and -ve oxidation states.
(d) Identify the element which neither exhibits -ve nor +ve oxidation state.
Solution.
(a) Fluorine (F) being most electronegative element shows only a -ve oxidation state of- 1.
(b) Cesium (Cs) is a highly electropositive alkali metal and has a single electron in the valence shell. Therefore it can exhibit an oxidation state of + 1.
(c) Iodine (I) because of the presence of seven electrons in the valence shell, shows an oxidation state of – I and because of the presence of d-orbitals it also exhibits +ve oxidation states of + 1, + 3, + 5 and + 7.
(d) Neon (Ne) is an inert gas and hence it neither exhibits -ve nor +ve oxidation states.
![]()
Question 23.
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for the reaction for this redox change taking place in water.
Solution.
The skeletal equation for the process would be:
Cl2(aq) + SO2(aq) + H2O(l) → 2Cl–(aq) + SO42-(aq)
Reduction half equation:
Cl2(aq) → Cl–(aq)
Balance Cl atoms,
![]()
Balance oxidation number by adding electrons:
Cl2(aq)+ 2e– → 2Cl–(aq) ……………………………………………. (i)
Oxidation half equation:
![]()
Balance oxidation number by adding electrons:
SO2(aq) → SO42-(aq) + 2e–
Balance charge by adding H+ ions:
SO2(aq) → SO42-(aq) + 4H+(aq) + 2e–
Balance O atoms by adding 2H2O,
SO2(aq) + 2H2O(l) → SO42-(aq) + 4H+(aq) + 2e– ……………………………………………. (ii)
Adding equation (i) and equation (ii), we get balanced redox reaction as,
Cl2(aq) + SO2(aq) + 2H2O(l) → 2Cl–(aq) + SO42-(aq) +4H+(aq)
Question 24.
Refer to the periodic table given in your NCERT book and now answer the following questions:
(a) Select the possible non-metals that can show disproportionation reaction. (6) Select three metals that show disproportionation reaction.
Solution.
(a) The non-metals are: phosphorus, chlorine and sulphur. The disproportionation reactions shown by them are given as follows:
(i) P4(s) + 3OH–(aq) + 3H2O(l) → PH3(g) + 3H2PO2–(aq)
(ii) Cl2(aq) + 3OH–(aq) → Cl–(aq) + CIO–(aq) + H2O(l)
(iii) S8(s) + 12OH–(aq) → 4S2(aq) + 2S2O32-(aq) + 6H2O(l)
(b) The metals are: copper, gallium and indium etc. The disproportionation reactions given by the metal ions Cu+, Ga+ and In+ are given below:
(i) 2Cu+(aq) → Cu2+(aq) + Cu(s)
(ii) 3Ga+(aq) → Ga3+(aq) + 2Ga(s)
(iit) 3In+(aq) → In3+(aq) + 2In(s)
Question 25.
In Ostwald’s process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.0 g of ammonia and 20.0 g of oxygen?
Solution.
The balanced equation for the reaction is:
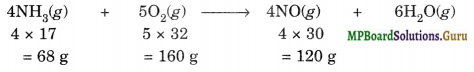
(I) Identifying limiting reagent
Here, 160 g of O2 will react with NH3 = 68 g
∴ 20 g of O2 will react with NH3 =\(\frac{68}{160}\) × 20 = 8.5 g
Thus,O2 is the limiting reagent, accordingly the amount of nitric oxide in the reaction would depend upon the amount of 02 taken and not on the amount of NH3 taken.
(II) Calculating amount of NO formed
160 g of O2 produce NO = 120 g
∴ 20 g of 02 will produce NO =\(\frac{120}{160}\) × 20 = 15 g.
Question 26.
Using standard electrode: Predict if the reaction between as the following is feasible.
(i) Fe3+ (aq) and I– (aq)
(ii) Ag+ (aq) and Cu
(iii) Fe3+ and Br~ (aq)
(iv) Ag and Fe3+ (aq)
(v) Br2 (aq) and Fe2+ (aq)
Given E°I2/I = 0.541 V, E°Cu2+/Cu = 0.34 V, E°Br2/Br–= 1.09 V, E°Ag+/Ag = 0.80 V,
Solution.
A redox reaction is feasible if its E° is positive
(i) Fe3+ (aq) + I– (aq) ⇌ Fe2+ (aq) + \(\frac{1}{2}\) I2
E°Redox = E°Reduced species – E°Oxidised species
= E°Fe3+/Fe2+ – E°I2/I
= 0.77 -0.54 = + 0.23 V (Feasible)
(ii) 2Ag+ (aq) + Cu ⇌ 2Ag(s) + Cu2+(aq)
E°Redox = E°Ag+/Ag – E° Cu2+/Cu
= + 0.80 – 0.34 = + 0.46 V (Feasible)
(iii) Fe3+ (aq) + Br– (aq) ⇌ Fe2+ (aq) + \(\frac{1}{2}\) Br2
E°Redox = E°Fe3+/Fe2+ – E° Br2/Br–
= 0.77 — 1.09 = – 0.32 V (Not Feasible)
(iv) Ag(s) + Fe3+ (aq) ⇌ Ag+(aq) + Fe2+(aq)
E°Redox = E°Fe3+/Fe2+ – E°Ag+/Ag
= 0.77 – 0.80 = – 0.03 V (Not Feasible)
(v) \(\frac{1}{2}\) Br2 (aq) + Fe2+ (aq)⇌ Br –(aq)+ Fe3+(aq)
E°Redox = E°Reduced species – E°Oxidised species
= E°Br2/Br– – E°Fe3+/Fe2+
= + 1.09 – 0.77 = + 0.32 V (Feasible)
Question 27.
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNOs with silver electrodes.
(ii) An aqueous solution of AgN03 with platinum electrodes.
(iii) A dilute solution of sulphuric acid using platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Solution.
(i) Anode Ag → Ag+ + e–
Cathode Ag+ + e– → Ag
(ii) Cathode Ag+ + e–→ Ag
Anode 2OH– → H2O +\(\frac{1}{2}\)O2 + 2e–
(iii) In dilute H2SO4 electrolysis of
H2O ⇌ H+ + OH–
Cathode 2H+ + 2e– → H2 ↑
Anode 2OH– → H2O +\(\frac{1}{2}\)O2 + 2e–
(iv) CuCl2 → Cu2+ + 2Cl–
Cathode Cu2+ + 2e– →Cu
Anode 2Cl– → Cl2 + 2e–
Question 28.
Arrange the following metals in the order in which they displace each other from their salts.
Al, Cu, Fe, Mg and Zn
Solution:
This is based upon the relative positions of these metals in the activity series.
The metal placed lower in the series can displace the metals occupying a higher position present as its salt. Based upon this, the correct order is:
Mg, Al, Zn, Fe, Cu.
Question 29.
Given the standard electrode potentials
K+/K = – 2.93 V, Ag+/Ag = 0.80 V Hg2+/Hg = 0.79 V; Mg2+/Mg = – 2.37 V, Cr3+/Cr= – 0.74 V
Arrange these metals in increasing order of their reducing power.
Solution:
If maybe noted that lesser the E° value for an electrode, more will be reducing power. The increasing order of reducing power is:
Ag < Hg < Cr < Mg < K.
Question 30.
Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place.
Further show:
(i) which electrode is negatively charged.
(ii) the carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Solution:
The galvanic cell for the reaction is
Zn(s)|Zn2+(aq) || 2Ag+(aq) 12Ag(s)
(i) Zinc electrode (anode) is negatively charged.
(ii) Current flows from silver to zinc in outer circuit
(iii) Anode: Zn(s) → Zn2+(aq) + 2e– (oxidation)
Cathode: 2Ag+(aq) + 2e– → 2Ag(s) (reduction)
![]()
MP Board Class 11 Chemistry Redox Reactions Important Questions and Answers
Question 1.
The formation of sodium chloride from gaseous sodium and gaseous chlorine is a redox process, justify.
Answer:
Na atoms get oxidised and chlorine is reduced.
Question 2.
When magnesium ribbon is burnt in air, two products are formed, magnesium oxide and magnesium nitride. Point out the oxidising and reducing agent.
Answer:
3Mg + N2 → Mg3N2 ; 2Mg + O2→ 2MgO.
Mg is reducing agent N2 and O2 are oxidising agents.
Question 3.
Give cathodic reaction involved in the electrolysis of acidified water.
Answer:
H2O + e– → OH– + \(\frac{1}{2}\) H2(g)
Question 4.
2A + H2SO4 → A2SO4 + H2. Give the representation of the cell which involves the above redox reaction.
Answer:
A/A +(aq) || H+(aq)/H2, Pt
Question 5.
What is meant by disproportionation? Give one example.
Answer:
A process in which same element undergoes increase as well as decrease in oxidation number.
Question 6.
A cell is set up by the two electrodes Cu/Cu2+ and Al/Al3+. What is the net cell reaction?
Answer:
2Al + 3Cu2+ → 2Al3+ + 3Cu
Question 7.
Standard electrode potentials of some elements are given as X( – 1.66 V), Y(+ 0.32 V), Z(- 3.00 V), E(+ 0.8 V) and G(- 0.76 V). Which one is the strongest oxidising agent and which one will be the strongest reducing agent?
Answer:
Strongest oxidising agent: E; Strongest reducing agent: Z.
Question 8.
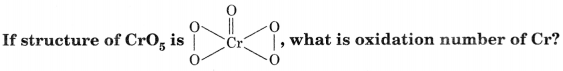
Answer:
Oxidation number of Cr = + 6
Four O atoms involved in peroxide linkage have O.N. = – 1 while the-fifth O atom has O.N. = – 2
Question 9.
What is the oxidation number of Pt in [Pt (C2H4) Cl3]–?
Answer:
x+0-3=-1
∴ x = + 4.
Question 10.
In the reaction given below which species is called spectator ion?
Zn + Cu2+ + SO42- → Zn2+ + Cu + SO42-.
Answer:
SO42- ions do not participate in reaction and thus, they are spectator ions.
Question 11.
A solution of silver nitrate was stirred with iron rod. Will it cause any change in the concentration of silver and nitrate ions?
Answer:
The concentration of Ag+ ions will decrease but NO3– ions will not have any change in concentration
2Ag+ + Fe → 2Ag + Fe2+.
Question 12.
Can we use KCl as electrolyte in the following cell Cu/Cu2+ || Al+/Al.
Answer:
KCl cannot be used in salt bridge in the given cell, because it will undergo chemical reaction with Ag+ ions to form a precipitate of AgCl.
Question 13.
What is oxidation numbers of C in HCN and HNC?
Answer:
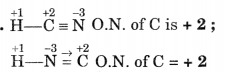
Question 14.
A layer of CuO on copper vessel can be easily cleaned by dilute HCl but oxide layer of aluminium on the aluminium vessel cannot be cleaned by HCl. Give reason.
Answer:
It is because Cu falls beneath hydrogen in the electrochemical series and hence copper from Cu+2 state can be easily reduced to Cu. On the other hand Al falls above H in electrochemical series and hence Al+3 cannot be reduced to Al.
![]()
Question 15.
An iron nail accidentally fell into a 1 M solution of sliver nitrate placed in glass vessel. What observations will be made?
Answer:
As reduction potential of Fe is less than Ag therefore Fe will get oxidised to Fe+3 whereas Ag+ will get reduced to Ag. In simple words the iron nail will get rusted.
Question 16.
Consider the following redox reactions that produce electricity in a galvanic cell:
(i) 2Fe3+ + 2Cl– → 2Fe2+ + Cl2(g)
(ii) Cd(s) + I2 → Cd2+ + 2I–
(iii) Cu2+ + Cr → Cu + Cr3+
Write the anode and cathode reactions for galvanic cell.
Answer:
(i) Anode: 2Cl– → Cl2 + 2e– ;
Cathode-. Fe3+ +e– → Fe2+
(ii) Anode: Cd → Cd2+ + 2e– ;
Cathode: I2 + 2e– → 2I–
(iii) Anode: Cr → Cr3+ + 3e– ;
Cathode: Cu2+ + 2e– → Cu
Question 17.
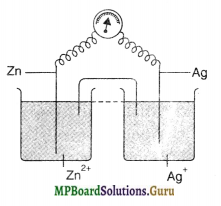
Draw the diagram for the galvanic cell which would have overall chemical reaction as
Zn + 2Ag+ → Zn2+ + 2Ag
Answer the following:
(i) Write the reactions occurring at each electrode.
(ii) In which directions do the electrons flow in the external circuit?
(iii) Name the salt to be taken in salt bridge.
(iv) How is the electrical neutrality maintained in the solutions of the two half cells?
(v) Label the anode and cathode.
(vi) How does the EMF change when the concentration of silver ions is decreased?
Answer:
(i) Anode: Zn → Zn2+ + 2e– ;
Cathode: Ag+ + e– → Ag
(ii) From Zn to Ag
(iii) KC1
(iv) By migration of ions through salt bridge (v) Zn (anode), Ag (cathode)
(vi) EMF decreases.
Question 18.
Starting with the correctly balanced half reactions, write the overall ionic reaction for the following changes:
(i) Chloride ion is oxidised to Cl2 by MnO4– in acid solution
(ii) Nitrous acid (HNO2) reduces Mn04″ in acid solution
(iii) Nitrous acid (HNO2) oxidises I– to I2 in acid solution
Answer:
(i) 2MnO4– + 10Cl– + 16H+ → 2Mn2+ + 8H2O + 5Cl2
Oxidation number of Mn changes from 7 to + 2
(ii) 5HNO2 + 2MnO4–+ 6H+→ 2Mn2+ + 5HNO3 + 3H2O
Oxidation number of N changes from + 3 to + 5
(iii) 2HNO2 + 2I– + 2H+ → 2NO + 2H2O + I2
Oxidation number of N changes from + 3 to + 2
Question 19.
(a) Describe the role of oxidation number in nomenclature.
(b) State which of the following is the strongest oxidising agent and why?
(i) MnO4–| Mn2+ E° = + 1.52
(ii) Fe3+ | Fe2+ E° = + 0.76
(iii) BrO3– | Br2 E° = + 1.50
(iv) Cr2O72- | Cr3+ E° = + 1.33
Answer:
(b) MnO4– is the strongest oxidising agent because it has highest reduction potential.
Question 20.
Some standard electrode potentials are given below:
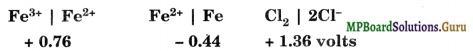
(i) Can Fe (II) act as an oxidising agent
(ii) When iron is reacted with Cl2, will it most likely form FeCl2 or FeCl3?
Answer:
(i) No, because its reduction potential is least
(ii) FeCl2 is preferred.
Question 21.
A student constituted a cell by the two electrodes Zn/Zn2+ (1 M) and Mg/Mg2+ (1 M) and represented it as Zn/Zn2+ (1 M) | | Mg2+ (1 M)/Mg. Is he correct or not?
(E°Mg+2/Mg = – -37 V; E°Zn+2/Zn = -0.76 V) Justify.
Answer:
The electrode with lower reduction potential will act as anode and the other with higher reduction potential acts as cathode. The representation of given cell is incorrect.