MP Board Class 11th Chemistry Important Questions Chapter 14 Environmental Chemistry
Environmental Chemistry Important Questions
Environmental Chemistry Objective Type Questions
Question 1.
Choose the correct answer:
Question 1.
Source of non – pollution energy:
(a) Fossil fuel
(b) Sun
(c) Gasoline
(d) Nuclear energy
Answer:
(b) Sun
Question 2.
Which radiations manufacture O3:
(a) Ultra – violet
(b) Visible
(c) Infra – red
(d) Radio waves
Answer:
(a) Ultra – violet
Question 3.
Which radiations provide Green house effect:
(a) Infra – red
(b) Visible
(c) Ultra – violet
(d) X – rays
Answer:
(c) Ultra – violet
![]()
Question 4.
PAN is responsible for:
(a) Depletion of ozone layer
(b) For smog
(c) For Acid rain
(d) Poisonous food
Answer:
(b) For smog
Question 5.
Which is not an air pollutant:
(a) H2
(b) H2S
(c) NO2
(d) O3
Answer:
(a) H2
Question 6.
Air pollutant released from jet aeroplanes in the form of an air pollutant:
(a) Photochemical oxidant
(b) Photochemical reductant
(c) Aerosol
(d) Physical pollutant
Answer:
(c) Aerosol
Question 7.
Is not present in Acid rain:
(a) H2SO4
(b) HNO3
(c) H2SO3
(d) CH3COOH
Answer:
(d) CH3COOH
![]()
Question 8.
Is responsible for disease of lungs:
(a) O2
(b) N2
(c) CO2
(d) SO2
Answer:
(d) SO2
Question 9.
O3 is manufactured in:
(a) Troposphere
(b) Stratosphere
(c) Mesosphere
(d) Thermosphere
Answer:
(b) Stratosphere
Question 10.
For acid rain ‘sink’ is:
(a) Leaves
(b) Reservoir
(c) Lime stone
(d) CO2
Answer:
(c) Lime stone
Question 11.
Primary pollutant is:
(a) SO3
(b) NO2
(c) N2O
(d) NO
Answer:
(d) NO
![]()
Question 12.
Most dangerous is:
(a) Smoke
(b) Dust
(c) Smog
(d) NO
Answer:
(c) Smog
Question 2.
Fill in the blanks:
- The air pollutant released by jet aeroplanes in the form of fluro carbon is …………………………..
- D.D.T. is …………………….. poisonous pollutant as compared to B.H.C.
- O3 is formed in the ……………………… level of atmosphere.
- A definite tolerable level of pollutants in the environment is expressed by …………………………….
- Maximum air pollutants are present in …………………………….. level of the atmosphere.
- Ozone layer prevent us from …………………………….. rays.
- Oxides of …………………………. and …………………………….. cause acid rain.
- …………………………… is the main cause of ozone layer depletion.
- ………………………….. gas is responsible for Green house effect.
- Substance produces pollution is known as ……………………………..
- ……………………………… is responsible for lung diseases.
- SO2 pollutant is responsible for the disease of ……………………………..
Answer:
- Aerosol
- More
- Stratosphere
- T.L.V.
- Troposphere
- Ultra – violet
- Nitrogen, sulphur
- C.F.C
- CO2
- Pollutant
- Photochemical smog
- Asthma
![]()
Question 3.
Answer in one word/sentence:
- Name the person who started Chipko Aandolan for the conservation of forest?
- Region of the atmosphere where living beings exist is known as?
- What is the name of compound responsible for hole in ozone layer?
- What is smoke containing fog known as?
- What is rain water containing small amount of sulphuric acid and nitric acid known as?
- Which chemistry produces environment friendly chemicals having minimum contribution in pollution?
- What is decrease in density of ozone gas due to chlorofluorocarbon compound in atmosphere known as?
- What is the maximum quantity of pollutant having no effect on receptor known as?
- When do we celebrate World Environment Day?
- What is PAN?
- Name the largest sink of earth?
- When does Bhopal Gas Tragedy occured?
- Name the disease due to water pollution?
Answer:
- Sunderlal Bahuguna
- Troposphere
- Chlorofluorocarbon
- Smog
- Acid rain
- Green chemistry
- Ozone hole
- Threshold limit value
- 5th June
- Peroxyacetyl nitrite/ photochemical smog
- Ocean
- Midnight of 2nd and 3rd December 1984
- Jaundice, Diarrhoea
Question 4.
Match the following:
[I]

Answer:
- (e)
- (c)
- (b)
- (d)
- (a)
[II]

Answer:
- (c)
- (d)
- (a)
- (b)
- (e)
[III]

Answer:
- (b)
- (a)
- (d)
- (c)
Environmental Chemistry Very Short Answer Type Questions
Question 1.
Write the definition of pollutants?
Answer:
Such substances whose amount in the environment increases more than required and cause harmful effect on human, animal and plant kingdom are called pollutants. Like: CO, CO2, NO, NO2, SO2 etc.
Question 2.
Write names of two air pollutants?
Answer:
SO2, SO3.
Question 3.
Name two pollutants which depletes ozone layer?
Answer:
- Cycle of nitric oxide (NO) and
- C.F.C. (Chlorofluoro carbon), in which C.F.C. is main.
Question 4.
Ozone is found in?
Answer:
Stratosphere.
![]()
Question 5.
Write the two health problems caused by SO2?
Answer:
- SO2 affects the respiratory canal and lungs due to which various health diseases are caused like cancer.
- Acid rain caused due to SO2 produces boils on the skin.
Question 6.
What is Acid rain?
Answer:
Various gaseous pollutant present in the atmosphere like SO2, SO3, NO2, NO dissolve in rain drop.
These drops fall with rain and are called acid rain.
SO2 + H2O → 2HSO3
SO3 + H2O → H2SO4.
Question 7.
Name any two green house gases?
Answer:
C.F.C. and CO2.
Question 8.
What is P.A.N.?
Answer:
It is peroxy acyl nitrate which is a photochemical smog.
Question 9.
What is C.F.C.?
Answer:
It is chlorofluoro carbon which is the main cause of depletion of ozone layer.
![]()
Question 10.
What is green chemistry?
Answer:
A technique to check pollution in which such chemical reactions are suggested which do not cause pollution and if pollution spreads then it can be destroyed. This is called green chemistry.
Question 11.
What is TLV?
Answer:
A definite value of pollutants can be tolerated. This is expressed by TLV. TLV means ‘Threshold Limit Value’.
Question 12.
What are particulate pollutants?
Answer:
The pollutants mixed up with air in liquid or solid state in such a manner that the remain suspended for a long time is called particulates.
![]()
Question 13.
What is specimasion?
Answer:
Many pollutants can be made from element. The method of determining which of the product is more dangerous is called specimasion.
Question 14.
What is sink?
Answer:
Sink is that in which the substance totally gets consumed and then also there is no effect on sink.
Question 15.
Name the biggest sink of the earth?
Answer:
The biggest sink of the earth is the sea.
Question 16.
What is green house effect?
Answer:
The heating of atmosphere due to absorption of infrared radiation by carbon dioxide and other gases is called green house effect.
Question 17.
Explain the mechanism of acid rain?
Answer:
All pollutant gases spread as pollutant particles in the form of smoke by the burning of fossil fuels and other fuels. Due to high temperature of industries and other engines, oxides of nitrogen spread in the atmosphere by the combination of N2 and O2. These gases mix in rain drops form acid and then fall on the earth as acid rain drops. This is called acid rain.
![]()
Question 18.
The gas which gets leak in 1984 in Bhopal is?
Answer:
CH3 – N = C = O Methyl isocyanate.
Question 19.
What is the main component for the depletion of ozone layer?
Answer:
Chlorofluorocarbon.
Question 20.
Name the person who started “Chipko Andolan” for forest conservation?
Answer:
Sunderlal Bahuguna.
Question 21.
Write the name of disease caused by water pollution?
Answer:
Cholera, Typhoid, Joindiss etc.
Question 22.
Name the medicinal plant which is helpful in controlling pollution and useful in skin diseases?
Answer:
Neem (Azaderecta indica).
Question 23.
Which gas is responsible for green house effect?
Answer:
Carbon dioxide (CO2).
![]()
Question 24.
What is the main sink of CO pollutant?
Answer:
Biological molecules present in soil.
Question 25.
Write the name of four methods useful in Green chemistry?
Answer:
- Use of sunlight
- Micro oven
- Micro waves
- Sound waves
- Use of enzyme.
Question 26.
Which pollutant is responsible for smog?
Answer:
PAN (Peroxy acyl nitrate).
Question 27.
What is sink for acid rain?
Answer:
Lime stone (Marble).
Question 28.
Which sphere is present near to earth?
Answer:
Troposphere.
Question 29.
Full form of B.H.C. is?
Answer:
Benzene Hexa chloride.
Question 30.
Which light is responsible for skin cancer?
Answer:
Ultra – violet light (UV – light).
![]()
Question 31.
What is responsible for lung diseases?
Answer:
Sulphur dioxide (SO2).
Question 32.
What is the main source of emmision of CO?
Answer:
Vehicles.
Question 33.
Oxides of which elements are responsible for acid rain?
Answer:
Oxides of nitrogen and sulphur.
Question 34.
Maximum pollution occurs in which sphere?
Answers:
Troposphere.
![]()
Question 35.
What is the main constituent for ozone depletion?
Answer:
C.F.C. (Chlorofluoro carbon).
Question 36.
Which radiation give green house effect?
Answer:
Infrared radiations (IR).
Question 37.
The formation of ozone takes place where in the atmosphere?
Answer:
Stratosphere.
Environmental Chemistry Short Answer Type Questions – I
Question 1.
Define Green Chemistry?
Answer:
Green chemistry is the branch of science in which study of effects of chemicals (like : Origin, transportation, reactions etc.) on environment is studied.
Question 2.
Explain Tropospheric pollution?
Answer:
Tropospheric pollution occurs due to unwanted solids and gas particles present in the air. The pollution occurs due to following two substances:
1. Gaseous air pollutants:
They are sulphur, nitrogen and CO2, H2S, hydrocarbon, ozone and other oxidising agents.
2. Particulates:
They are dust, fog, smoke etc.
![]()
Question 3.
Which gases are responsible for green house effect?
Answer:
Main gases are CO2, methane, water vapour, nitrous oxide, chlorofluoro carbon (CFC) and ozone.
Question 4.
What do you mean by BOD (Biochemical oxygen demand)?
Answer:
The total amount of oxygen consumed by microorganism in decomposing the wastes present in a certain volume of a sample of water.
Question 5.
Due to green house effect temperature of the earth is increasing? Which substances are responsible for it?
Answer:
Green house gases like CO, methane, nitrous oxide, ozone and chlorofluoro carbons are responsible for green house effect.
Question 6.
Ozone is a toxic gas and is a strong oxidizing agent even then its presence in the stratosphere is very important Explain what would happen if ozone from this region completely removed?
Answer:
The ozone layer acts as a protective umbrella and does not allow the harmful UV radiations to reach the earth’s surface.(MPBoardSolutions.com) If ozone is completely removed from the stratosphere, the UV radiations will fall directly on the humans, causing skin cancer and on the plants affecting plant proteins.
Question 7.
What are the sources of dissolved oxygen in water?
Answer:
- Photosynthesis
- Natural aeration
- Artificial aeration.
![]()
Question 8.
Dissolved Oxygen in water is very important for aquatic life. What processes are responsible for the reduction of dissolved oxygen in water?
Answer:
Dissolve oxygen is essential for sustaining animal and plant life in any aquatic system. The wastes such as domestic, industrial and biodegradable organic compounds are oxygen demanding wastes. These are decomposed by the bacterial population which in turn decreases the oxygen from water.
Question 9.
What are Biodegradable and non – biodegradable pollutants?
Answer:
- Biodegradable pollutants: They can be degrade by microorganisms.
- Example: Sewage, dungs of animals, fruits and vegetable peels etc.
- Non – biodegradable pollutants: They cannot degrade by microorganisms.
- Example: Mercury, Lead, DDT, glass, plastic etc.
Question 10.
What is pollution?
Answer:
Environmental pollution is the effect of undesirable changes in our surroundings that have harmful effects on plants, animals and human beings.
Question 11.
What is pollutant?
Answer:
A substance present in the environment in greater proportion than its natural abundance and resulting into harmful effects, is called a pollutant.
Question 12.
What are contaminants?
Answer:
Some substances which are not present in the environment, but are released in the environment as a result of chemical activities lead to pollution. Such substances are called contaminants. Example: Methyl isocyanate gas (CH3NCO).
Question 13.
Write the chemical name of the gases depleting ozone?
Answer:
Nitric oxide, atmospheric oxygen and chlorofluoro carbon are responsible.
![]()
Question 14.
Which are green house gases?
Answer:
CO2, ozone and water vapours are green house gases. They have the tendency to absorb IR radiations.
Question 15.
What is polluted air?
Answer:
If some underisable substances get added in the air which affects the health of the organisms, then such air is called polluted air.
Question 16.
Why there is ozone depletion over Antarctica?
Answer:
In the stratosphere compounds formed are converted back into chlorine free radical which deplete ozone layer.
Question 17.
What is the importance of BOD measurement of any water sample?
Answer:
BOD is the measurement of pollution caused by organic biodegradable substances in water sample. The less value of BOD tells that less amount of organic effluent is present in water.
Question 18.
Oxidation of sulphur dioxide into sulphur trioxide in the absence of a catalyst is a slow process but this oxidation occurs easily in the atmosphere. Explain how does this happen. Give chemical reaction for the conversion of SO2 into SO3
Answer:
The oxidation of sulphur dioxide into sulphur trioxide can occur both photochemically or non – photochemically. In the near ultraviolet region, the SO2 molecules react with ozone photochemically.
SO2 + O3 \(\underrightarrow { h\nu } \) SO3 + O2
2SO2 + O2 \(\underrightarrow { h\nu } \) 2SO3
Non – photochemically, SO2 may be oxidised by molecular oxygen in presence of dust and soot particles.
2SO2 + O2 \(\underrightarrow { Particulates } \) 2SO3
![]()
Question 19.
How is ozone formed in stratosphere?
Answer:
Ozone in the stratosphere is a product of (UV) radiations acting on dioxygen (O2) molecules. The (UV) radiations split apart molecular oxygen into free oxygen (O) atoms. These oxygen atoms combine with the molecular oxygen to form ozone.
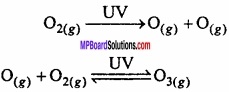
Question 20.
What is chlorosis?
Answer:
Chlorophyll in plants is formed slowly. This is due to presence of SO. This pollution is called chlorosis.
Question 21.
What is Metathesis?
Answer:
Metathesis is the name of that science, in which the study of application of chemical methods for general persons is studied.
Question 22.
What are primary and secondary air pollutants?
Answer:
Primary air pollutants are those which remain as such after their formulation e.g. NO, while secondary pollutants are formed as a result of chemical interaction between primary air pollutants. Example: PAN.
Question 23.
What is photochemical smog?
Answer:
Photochemical smog:
It is formed by photochemical reactions involving solar radiations. The principal constituents are O3, NO2 and some photochemical oxidants. It is also called Los Angeles smog.
Question 24.
What is acid rain?
Answer:
Acid rain:
It is the rain water containing sulphuric acid, nitric acid and small amount of hydrochloric acid which are formed from the oxides of sulphur and nitrogen present in the air as pollutants and has a pH of 4 – 5.
CO2 + H2O → H2CO3
SO3 + H2O → H2SO4
Question 25.
When CO2 is called harmful gas?
Answer:
Normal amount of CO2 in atmosphere is not harmful. Whereas organisms and plants prepare their food with the help of it. But, when the amount of CO2 increases due to various process then it alters the environmental balance and become harmful.
![]()
Question 26.
What is the role of CO2 in the “Green house effect”?
Answer:
Water vapours are present only near the earth atmosphere but ozone found very far from the earth and CO2 is found everywhere in the atmosphere. (MPBoardSolutions.com) So for green house effect, CO2 is more responsible because CO2 has the tendency to absorb IR radiations. Due to this green house effect produced.
Question 27.
Why acid rain is harmful for Tajmahai?
Answer:
Tajmahai is made up of marbles (CaCO3). Acid rain contains H2SO4 in dilute state. It reacts with marble of Tajmahai and make it discoloured and lustreless.

Question 28.
What is Fly ash pollution?
Answer:
The smaller ash particles are formed when fossil fuel is burnt. Gases produced during burning take the ash particles and pollute the atmosphere. The pollution is called fly ash pollution.
Question 29.
What is contaminated water? Name of diseases occurs due to it?
Answer:
The water which contains dissolved organic matters and salts and micro – organisms is called contaminated water.
Diseases are:
- Diarrhoea
- Typhoid
- Skin diseases etc.
Question 30.
What is Global warming?
Answer:
This increase in average temperature of global air due to increased green house effect is called ‘global warming’.
Question 31.
What is Ionosphere?
Answer:
It is also 40 km thick layer at an altitude of 50 km from the earth surface. It is also called mesosphere. Various ionic reactions taking place in ionosphere is:
O2 + \(\overset { \bullet }{ O } \) → O2+ + O
O+ + N2 → NO+ + N
N2+ + O2 → N2 + O2+
Environmental Chemistry Short Answer Type Questions – II
Question 1.
How is the poisonous effect of CO produced on man and animals?
Answer:
CO has poisonous effect on man and plants.
1. It combines with haemoglobin of the blood more strongly than oxygen.
CO + Hb → CO – Hb (Carboxy haemoglobin).
As a result of this amount of haemoglobin available in the blood for the transport of oxygen to the body cells decreases. The normal metabolism is thus, impaired due to less O2 level. This will cause suffocation and will ultimately lead to death. (MPBoardSolutions.com) Carbon monoxide if present in air can cause mental impairment, respiratory problems, muscular weakness and dizziness.
2. A high concentration of CO (100 ppm or more) will harmfully affect the plants causing leaf drop; reduction of leaf size and premature aging etc.
![]()
Question 2.
Write the harmful effects of SO2 (Sulphur dioxide)?
Answer:
Harmful effects of SO2 are:
1. SO, affects respiratory tract producing nose, eye and lung irritation. It has been reported that lower concentration of SO2 causes respiratory weakness.
If present at a concentration of only 2.5 ppm in the environment, then also it leads to dangerous diseases like bronchitis and lung cancer etc.
2. SO3 produce harmful effect on buildings made of marble and lime stones (CaCO3). The gas released from Mathura oil refinery is harmful for the Tajmahal.
3. High concentration of SO2, leads to stiffness of flower buds which eventually fall off from plants.
4. Air polluted by oxides of sulphur enhances the corrosion of metals like copper, zinc, iron etc.
Question 3.
Write the harmful effects of nitrogen dioxide?
Answer:
Nitrogen dioxide is harmful and poisonous. It produces the following harmful effects:
- It reacts with the ozone present in the atmosphere and decreases its density.
- Oxides of nitrogen are responsible for the production of photochemical smog.
- Oxides of nitrogen cause harmful effects on textile fibres like nylon, rayon, cotton fibres etc. NO2 causes cracks in rubber.
- Increase in concentration of NO2 in the atmosphere is harmful for plants. It leads to leaf spotting, retards photosynthetic activity, retards plant growth etc.
- NO2 creates problems in human respiration and leads to bronchitis.
Question 4.
A farmer was using pesticides on his farm. He used the product of his farm as food for rearing fishes. He was told that fishes were not fit for human consumption because large amount of pesticides had accumulated in the tissues of Fishes. Explain, how did this happen?
Answer:
Pesticides are organic compounds which are used to protect plants from pests. These are mild poisons. These pesticides stick to the plants and also flow into lakes along with the rain water. (MPBoardSolutions.com) Rearing fishes when consume these plants as their food the poisonous pesticides accumulate in the tissues of fishes. Thus, these fishes are not fit for human consumption.
![]()
Question 5.
For dry cleaning, in the place of tetrachloroethene, liquefied carbon dioxide with suitable detergent is an alternative solvent. What type of harm to the environment will be prevented by stopping use of tetrachloroethane? Will use of liquefied carbon dioxide with detergent be completely safe from the point of view of pollution? Explain?
Answer:
1. Tetrachloroethene (Cl2C = CCl2) is suspected to be carcinogenic and contaminates the ground water. This harmful effect will be prevented by using liquefied CO2 along with suitable detergent.
2. Use of liquefied CO2 along with detergent will not be completely safe because detergents also cause pollution as most of the detergents are non – biodegradable. Also, liquefied CO2 will ultimately enter into the atmosphere and contribute to the green house effect.
Question 6.
What are the harmful effect of Green house effect?
Answer:
Though green house effect was beneficial in maintaining a livable temperature on earth. But excessive CO2 in the atmosphere due to deforestation and large scale burning of fossil fuel has disturbed the natural balance in favour of higher green house effect. This has led to an increase in average temperature of the earth from 0.3 to 0.6°C over the past century. This increase in average temperature of global air due to increased green house effect is called ‘Global warming’.
The atmospheric CO2 level is expected to become double sometimes between 2050 – 2150 with a corresponding increase in global temperature from 1 to 3°C. Besides CO2, other green house gases are methane, water vapour, nitrous oxides, CFCs (Chlorofluorocarbons) and ozone. Methane is produced naturally when vegetation is burnt, digested or rotted in the absence of oxygen. (MPBoardSolutions.com) Large amount of methane are released in paddy fields, coal mines, from rotting garbage dumps and by fossil fuels.
CFCs are man made industrial chemicals used in air conditioning etc. CFCs are also damaging the ozone layer. Nitrous oxide occurs naturally in the environment. In recent years, their quantities have increased significantly due to use of chemicals, fertilizers and the burning of fossil fuels.
Harmful Effects of Global Warming:
- There will be rise in sea level due to increased rate of melting of glaciers. Sea level may rise by 0.5 to 1.5 m during the next 50 to 100 years if present rise in CO2 continues. This will result in flood and loss of soil particularly in coastal areas.
- Higher global temperature is likely to effect the whole ecosystem by disturbing the life cycle of certain micro and macro organisms.
- Higher temperature is likely to increase incidence of infectious diseases such as malaria, dengue, yellow fever and sleeping sickness.
Question 7.
Write the reasons of water pollution?
Answer:
The sources of water pollution are as follows:
- Organic pollutants like manure wastes from food processing, rags, paper discards etc.
- Industrial wastes.
- Detergents and Fertilizers: The detergents are best available mode for the growth of bacteria.
- Pollution of water takes place through acids.
![]()
Question 8.
How is artificial green house prepared?
Answer:
Synthetic green house:
In nature, coating of CO2 is forming green house, but synthetic green house can be synthesized by studying its mechanism.
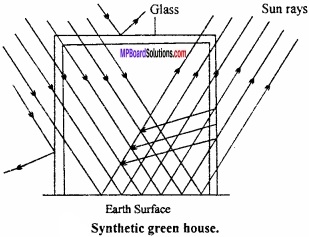
Actually the transparent glass roof and wall of the glass house allow sun rays to pass through and strike the surface of the house. The reflected radiation is of longer wavelength than the incident radiation. A significant portion of reflected radiation absorbed by glass. (MPBoardSolutions.com) As radiation of longer wavelength (Infrared radiation) generates heat, this causes rise in temperature inside the glass house. An effect similar to one in glass house is responsible for keeping the earth’s surface warmer.
Question 9.
For your agricultural field or garden you have developed a compost producing pit Discuss the process in the light of bad odour, flies and recycling of wastes for a good produce?
Answer:
The compost is very useful for agriculture as a fertilizer. But the compost pro-ducing pits may give bad odour and flies. Therefore, the compost producing pit should be set up at a suitable place or in a bin to protect ourselves from bad odour and flies. It must be kept covered so that flies cannot enter into it and there is not much a bad odour.
Question 10.
How house effluents can be used as manure?
Answer:
Waste Management of Household waste:
All the solid household waste should be put in the household garbage box/bin. This should be then put into the community bins so that the municipal workers can take it in their vehicles to the disposable site. Here, the garbage is separated into biodegradable and non – biodegradable materials. (MPBoardSolutions.com) The biodegradable waste is deposited in the land fills. With the passage of time, it is converted into manure compost. Remember that if the waste is not collected in the garbage bins, it may find its way to sewers and some may to eaten up by the cattle.
The non – biodegradable waste like polythene bags, metal scrap, etc. choke the sewers. The polythene bags, if swallowed by cattle, can result into their death. The best way to manage domestic waste is to keep two garbage bins, one for the biodegradable (Non – recyclable) and the other for non – biodegradable (Recyclable) which can be sold to the vendor/dealer.
Question 11.
What do you mean by green chemistry? How will it help in decrease environmental pollutions?
Answer:
By green chemistry we mean a strategy to design chemical process which neither use toxic chemicals nor release the same to the atmosphere. It also means to develop methods of using raw materials more efficiently and generating less wastes.
The creative and innovative skills of green chemistry has developed many new environmental friendly processes, analytical tools, reaction conditions and catalysts etc. A few of these achievement may be listed as follows:
- Development of new method to improve the yield of ibuprofen upto 99%.
- Chlorofluorocarbon used as blowing agents for polystyrene foam (Thermocol) sheets have been replaced by CO2.
- A new technique of catalytic dehydrogenation of ‘diethanolamine’ produces an environment friendly herbicide. This process has avoided the use of highly toxic cyanide and formaldehyde.
- Organotin: A common antifouling compound used by sea marines has been replaced by a rapidly degradable compound called ‘sea nine’.
![]()
Question 12.
Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Answer:
Carbon monoxide is highly poisonous in nature. It combines readily with haemoglobin (It has more affinity than oxygen). Due to the formation of carboxyhaemoglobin, the quantity of oxygen to the body cell get reduced i.e. (MPBoardSolutions.com) CO reduces the oxygen carrying capacity of the blood and this leads to oxygen starvation (Anoxia). The deficiency of oxygen produces headache, dizziness, choking cardiac and pulmonary complications leading to paralysis and death. CO2 does not combine with haemoglobin. However, it is a green house gas and helps in global warming. Hence, it is less dangerous pollutant.
Question 13.
What would have happened if the green house gases were totally missing in the earth’s atmosphere? Discuss?
Answer:
The solar energy radiate back from earth surface is absorbed by the green house gases (i.e. CO2, CH4, O3, CFC and water vapour) present near the earth surface. Thus, they heat up the atmosphere near the earth’? surface and keep it warm. (MPBoardSolutions.com) As a result, they keep the temperature of the earth constant and help in the growth of plants and existence of life on the earth. If there were no green house gases, there would have no vegetation and life on the earth.
Question 14.
Statues and monuments in India are affected by acid rain. How?
Answer:
Statues and monuments are generally made of marble (Taj Mahal). The acid rain contains H2SO4 which attacks the marble.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
As a result, the monuments (Taj mahal) are being slowly corroded and the marble is getting discoloured and lustreless.
![]()
Question 15.
What are the harmful effect of water pollution? How they can be controlled?
Answer:
The harmful effects of water pollution are:
- Due to intake of polluted water many diseases like typhoid, dysentry etc. occur.
- Due to effluents the amount of dissolved oxygen in water decreases.
- Due to presence of soap and detergent effluents the water become poisonous for fishes.
Control of water pollution:
We have seen that the two sources of water pollution are: Sewage and industrial wastes. They should be removed from water before it is put to use.
Treatment of sewage:
1. Sewage must be churned by machines so that the large pieces may break into smaller ones and may get mixed thoroughly. The churned sewage is passed into a tank with a gentle slope. Heavier particles settle and the water flowing down is relatively pure.
2. Water must be sterilized with the help of chlorination. It kills microbes of sewage fungus as well as some pathogens, spores or cytes. Chlorination is very essential particularly in rainy season.
3. Treatment of water with alum, lime etc, also helps in its purifications.
Treatment of industrial wastes:
The treatment of industrial waste depends upon the nature of the pollutants present. In order to ascertain it, the pH of the medium is first determined and the wastes is then neutralised with the help of suitable acid or alkalis. (MPBoardSolutions.com) The chemical substances present in the industrial wastes dissolve in water can be precipitated by suitable chemical reaction and removed later on from water quite recently. Photocatalysed and ion – exchanges have been developed for the treatment of industrial wastes.
![]()
Question 16.
What is soil pollution? Write down methods of prevention of soil pollution?
Answer:
Soil pollution:
Change in physical and chemical property of soil due to humans and natural cause is known as soil pollution.
Soil pollution can be prevented by the following methods:
- Solid and unusable substances like iron, copper, glass, polythene, etc. should not be hurried under soil.
- Banning cutting of forest and uncontrolled grazing. Crop cycle to be adopted. Suitable arrangement for irrigation to be made. Control on flood and appropriate use of chemical fertilizers and insecticides.
- Minimum use of chemical fertilizers, insecticides and pesticides.
- Special attention on recycling of solid waste on melting.
- Emphasis on the use of cow – dung and human excreta as bio – gas.
- Biological insecticides to be used.
- Using closed mines for disposal off waste.
- Methods of soil erosion to be checked.
- Soil management to be adopted.
- Encouraging the use of biofertilizers.
Question 17.
Write the effects of depletion of ozone layer?
Answer:
Sunlight contains ultraviolet radiations and ozone layer present in the atmosphere prevents ultraviolet radiation to reach the earth surface. Continuous depletion of ozone layer cannot prevent ultraviolet radiations from reaching the earth surface and following disadvantages will occur.
- Intensity of sunlight will increase and temperature of the environment will become intolerable.
- Increase in skin diseases.
- Skin cancer becomes common.
- Immune system will turn weak.
- Germination and development of seed slows down.