MP Board Class 11th Chemistry Important Questions Chapter 13 Hydrocarbons
Hydrocarbons Important Questions
Hydrocarbons Very Short Answer Type Questions
Question 1.
Full form of T.E.L?
Answer:
Tetra Ethyl Lead.
Question 2.
What is obtained when ethylene dibromide is heated with Zn dust?
Answer:
Ethylene.
Question 3.
The smelling substance in L.P.G is?
Answer:
Ethyl mercaptan.
![]()
Question 4.
What is the name of the method in which by electrolysis of potassium acetate, methane is formed?
Answer:
Kolbe’s reaction.
Question 5.
Write the name of the reaction
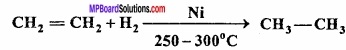
Answer:
Sabatier and Senderens reaction
Question 6.
Write the names of the products formed in following reaction:
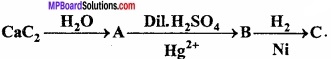
Answer:
(A) CH E= CH
(B) CH3 – CHO
(C) CH3 – CH2 – OH
Question 7.
Write the reaction in which alkane is formed by alkyl halide and sodium
Answer:
Wurtz – Fittig reaction
Question 8.
What is number of a and n bonds in ethene?
Answer:
5o and 1 n.
![]()
Question 9.
In benzene, carbon has which hybridization?
Answer:
sp2 hybridization
Question 10.
In HC = CH, which hybridization is present in C – C?
Answer:
sp – sp2 hybridization
Hydrocarbons Short Answer Type Questions – I
Question 1.
Trans – alkene is formed by the reduction of alkyne by liquor ammonia. Is butene show geometrical isomerism obtained by reduction of 2 – butyne?

2 – butene shows geometrical isomerism.
Question 2.
Despite their I – effect, halogens are o – and p – directing in haloarenes. Explain?
Answer:
Halogen have (-1) and (+R) effect, these groups are deactivating due to their (-1) effect and they are ortho and para directing due to (+R) effect.
Question 3.
Alkenes are more reactive than alkanes. Why?
Answer:
In alkanes there is only σ – bond between C – C but in alkenes there is one and one σ – bond between C = C. Due to lateral overlapping the π – bond is weaker than the σ – bond. Hence, alkenes are more reactive than alkanes. (MPBoardSolutions.com) The bond energy of π – bond lower than the bond energy of π – bond. Due to this difference of bond energy alkenes are more reactive as compared to alkanes.
Question 4.
What are asymmetric carbon?
Answer:
The carbon atom in which four different groups are attached is called asymmetric carbon. Due to this property, compound shows optical activity.
![]()
Question 5.
What do you mean by Chirality?
Answer:
Those molecules which are not superimposable on their mirror images are chiral molecules and this property is called chirality. They are optically active. The Chirality due to presence of asymmetric carbon in the molecule.
Question 6.
What are Alkanes? Which type of bonds are present in it?
Answer:
Alkanes are saturated hydrocarbons because of their low reactivity, they are also called paraffin. The general formula is CnH2n+2, In alkanes each carbon atom is sp3 hybridized. Single σ – bonds are present between C – C and C – H.
Example: Methane CH4, Ethane C2H6.
Question 7.
What are Alkenes? In Alkenes C is present in which hybridized state?
Answer:
A saturated hydrocarbon becomes unsaturated when two hydrogens are less in it. Such hydrocarbons are called olefins. In IUPAC system these olefins are called alkenes. Such alkenes are unsaturated and they contain C = C double bond. General formula is
The hybridization of carbon in C = C is sp2.
Example: Ethene CH2 = CH2, Propylene CH3 – CH = CH2.
Question 8.
What are Alkynes? What type of bonds are present in them?
Answer:
Decrease in four hydrogens in saturated hydrocarbons result in the formation of triple bonds between two carbon atoms. The unsaturated hydrocarbon produced known as alkynes. (MPBoardSolutions.com) Their general formula is CnH2n-2. Carbon atom of alkyne is sp3 hybridized. Alkynes are also called acetylenes. They contain carbon – carbon triple bond.
Example: Acetylene CH2 = CH2, Propyne CH3 – C = CH2.
![]()
Question 9.
Why Cyclopropane is more reactive than cyclohexane?
Answer:
In cyclopropane the bond angle in C – C – C is 60° due to which ring feelstrain, and so it is reactive and less stable. Whereas in cyclohexane C – C – C have 109°28 and have less strain in the ring, therefore it is stable and less reactive.
Question 10.
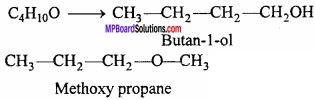
Explain functional isomerism with example?
Answer:
The compound having same molecular formula but different functional groups in the molecule are called functional group isomers.
Example:
1. Alcohols and ethers (C2H2n+2 – O)
Question 11.
What is dissociation?
Answer:
The method of separation of dor l image isomers from racemic mixture is called dissociation. This is done through biochemical or chemical methods.
Question 12.
How will you obtain nitrobenzene from acetylene?
Answer:
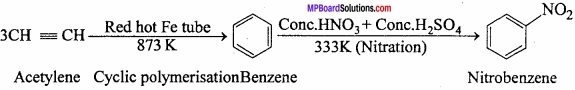
Question 13.
What is Prototrophy?
Answer:
By hydration of Propyne, enol and keto forms are obtained. It shows tautomerism and this type of isomerism is present in that compounds which have at least one hydrogen. This isomerism is due to the transfer of proton from one place to another. This is called prototrophy.
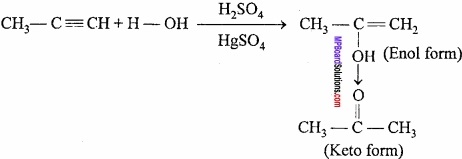
Question 14.
What is conformational stereioisomerism?
Answer:
The phenomenon of easily interconvertibility at room temperature due to free rotation around the carbon – carbon single bond in alkanes and its derivatives is called conformational stereioisomerism.
Question 15.
Explain the process of polymerisation?
Answer:
In polymerisation many simple molecules of a substance combine together to form a big molecule. The simple molecule is called monomer and the big molecule as polymer or macromolecule. Rubber, nylon, bakelite, P.V.C. are examples of high polymers. (MPBoardSolutions.com) Polymerisation of alkenes takes place in presence of Lewis acid BF3, AlCl3 or organic and inorganic peroxides.

Polyethylene is used as electrical insulator and as packing materials.
Hydrocarbons Short Answer Type Questions – II
Question 1.
What is Cracking? Write a note on cracking and its uses?
Answer:
Cracking or Pyrolysis:
Higher hydrocarbons when heated to high temperature decomposes into smaller hydrocarbons (lower carbon atom containing molecule). This type of thermal decomposition is known as cracking or pyrolysis.
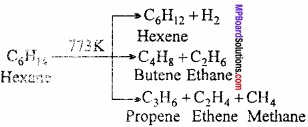
Pyrolysis is related to free radical reaction. Manufacture of oil gas or petrol gas is based on the concept of pyrolysis. For example, dodecane when heated to 973 K gives a mixture of heptane and pentene. Platinum, palladium or nickel is used as catalyst.
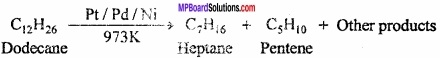
Steam phase cracking, catalytic cracking and liquid phase cracking are the different methods of cracking.
Uses of alkanes:
- Alkanes are used for making carbon black which is used for making ink, black paints and polish.
- As a gaseous fuel in industries and as L.P.G.
- Higher alkanes like petrol, kerosene, lubricant, oil, paraffin, wax, etc.obtained from petroleum are useful.
- Some halogen derivatives like chloroform and carbon tetrachloride are useful in laboratories and industries.
- Catalytic oxidation of alkanes give important compounds like alcohol, aldehyde, acid, etc.
![]()
Question 2.
Explain Dehydrohalogenation and Dehalogenation with example?
Answer:
Dehydrohalogenation:
When alkyl halide is heated with alcoholic KOH, molecule of hydrogen halide is eliminated forming alkene. This reaction is known as dehydro – halogenation.
Example:
CH3 – CH2 – CH2 – Cl + KOH CH3 – CH = CH2 + KCl + H2O.
Dehalogenation:
Vicinal dihalogen derivatives of alkanes are compounds in which halogen atoms are present on adjacent carbon atoms. They are also called, 1,2 – dihalogen derivative. They form alkenes when heated with Zn dust.
This reaction is known as dehalogenation:
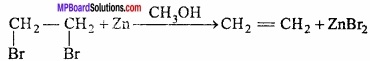
Question 3.
Write notes on Friedei – Crafts reaction?
Answer:
Alkylation:
When benzene or its higher homologous are heated with alkyl halide in presence of anhydrous AlCl3 alkyl derivatives of benzene are formed.

Acetylation:
When benzene or its higher homologous are treated with acid chloride in presence of anhydrous AlCl3, acyl benzene or aromatic ketones are formed.

Question 4.
What is Lindlar’s Catalyst? Write its uses?
Answer:
Lindlar catalyst is a palladium supported mixture of calcium carbonate and poisoned with sulphur or quinoline.
Uses:
Aikynes react with hydrogen in presence of nickel powder or platinum or palladium catalyst to form alkenes. This process is known as catalytic hydrogenation.
Question 5.
Geometrical isomerism is found in which type of compounds? Explain with example?
Answer:
Geometrical isomers and Geometrical isomerism or cis – trans isomerism:
This type of isomerism is shown by those alkene derivatives in which different groups are attached with carbons linked with double bond. (MPBoardSolutions.com) For example: compound like abc = cba. When similar atom or group is on same side of bond, it is called cis – isomer and when they are on opposite side of bond, it is called trans – isomer. This type of isomerism is called cis – trans isomerism.
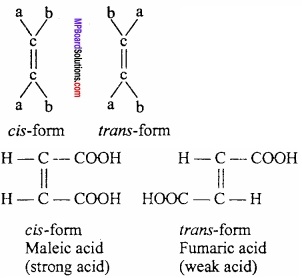
Following compounds does not show geometrical isomerism.
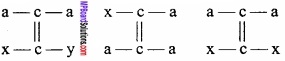
Question 6.
What are Dienes, write their types? Explain with example?
Answer:
Diene is an unsaturated hydrocarbon. In this, two double bonds are present between carbon – carbon chain. On the basis of position of double bonds dienes are of three types:
1. Isolated dienes:
Dienes in which more than one single bonds are present between two double bonds in C – C chain.
CH2 = CH – CH2 – CH = CH2
2. Conjugated dienes:
In these dienes the double bonds are present in alternate position in carbon – carbon chain.
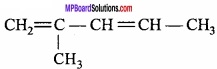
3. Cumulative dienes:
In these dienes the double bonds are present continuously on two C atoms.
CH3 = C = CH – CH3
CH3 – CH = C = CH2.
Question 7.
Write Diel’s – Alder reaction with equation?
Answer:
When a conjugated diene is heated with ethene, then a cyclic compound is formed. This is called Diel’s – Alder reaction.
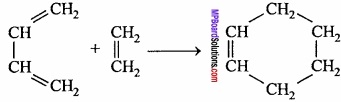
Question 8.
What is Huckel’s Rule?
Answer:
According to it, all those planar cyclic compounds exhibit aromatic character, whose ring contain (4n + 2) n electrons. Where n is an integer. Therefore, planar cyclic compounds in which there are 2 (n = 0), 6(n = 1), 10 (n = 2), 14 (n = 3) π electrons exhibit aromatic property.
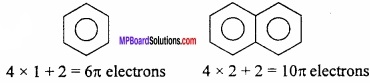
Benzene and Naphthalene are aromatic compounds on the basis of Huckel’s rules several heterocyclic compounds should also show aromatic properties.
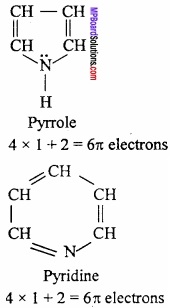
Question 9.
Write Kolbe’s method for manufacuture of alkanes?
Answer:
When sodium or potassium salt of carboxylic acids are electrolysed, alkanes are obtained at anode.
CH3COONa ⇄ CH3COO– + Na+
At anode:
CH3COO– – e– → CH3COO\(\overset { \bullet }{ C } \)
CH3 – COO. → \(\overset { \bullet }{ C } \)H3 + CO2
\(\overset { \bullet }{ C } \) H3 + \(\overset { \bullet }{ C } \) H3 → C2H6
At cathode:
Na+ + e– → Na
2Na + 2H2O → 2NaOH + H2
Question 10.
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitution reactions with difficulty?
Answer:
The orbital structure of benzene shows that the π electrons cloud lying above and below the benzene ring is loosely held and therefore it is likely to be attacked by electrophiles which subsequently bring about substitution. The nucleophiles would be repelled by the n – electron ring and hence benzene ring reacts with nucleophiles with difficulty.
![]()
Question 11.
Why do alkenes prefer to undergo electrophilic addition reactions while arenes prefer electrophilic substitution reactions? Explain?
Answer:
Alkenes are source of loosely held π – bonds. Due to which they show electrophilic addition reactions. There occurs a tremendous change in the energy during the electrophilic addition of electrons to alkenes therefore they show electrophilic addition reactions.
In Arenes, during the electrophilic addition reactions the aromatic nature of benzene destroyed, but in electrophilic substitution reactions it remains constant. (MPBoardSolutions.com) So the electrophilic substitution reactions are more stable in order of energy.
Question 12.
What is tautomerism? Explain with example?
Answer:
Tautomerism:
This is a special type of functional isomerism in which the isomers differ in the arrangement of atoms but they exist in dynamic equilibrium with each other. For example, acetaldehyde and vinyl alcohol are tautomers which exist in equilibrium as:
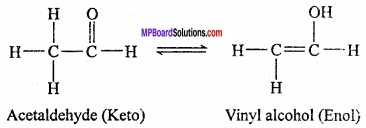
It is of different types but the most common among them is the keto – enol tautomerism. This arises due to 1, 3 – migration of a hydrogen atom from one polyvalent atom to other within the same molecule.
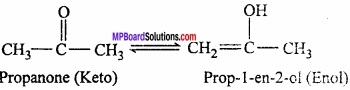
Conditions for the molecule to show tautomerism:
1. Presence of electron withdrawing groups such as:
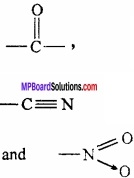
2. Presence pf α – hydrogen:
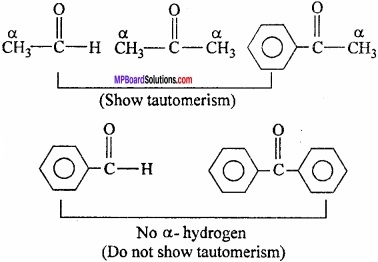
Question 13.
What is Newman projection formula?
Answer:
Newman projection:
Named after M.S. Newman, who first proposed this method of representing the three – dimensional structure on paper, this is easier to visualize than the one described before. In this projection, the molecule is viewed at the C – C bond head on. (MPBoardSolutions.com) In this formula, front carbon atom is shown by dot and rear carbon atom by a circle. Three hydrogen atoms bonded to the carbon atoms are shown by lines making an angle of 120° with each other.
Newman’s projection formula of ethane shown in fig. gives a planar representation for two – dimensional (2 – D) representation of the molecule. (MPBoardSolutions.com) Staggered form changes into eclipsed form when rotated through 60°. Similarly, eclipsed form also changes into staggered form.
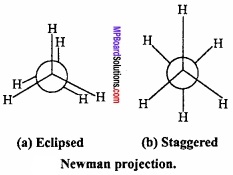
Question 14.
What is Markownikoff s rule?
Answer:
During the addition across unsymmetrical double bond, the negative part of the adding molecule attaches itself to the carbon atom carrying less number of hydrogen atoms.
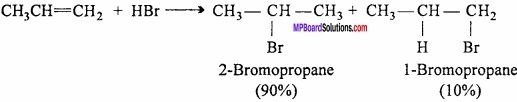
Markownikoffs rule can be explained on the basis of the mechanism of addition reaction. Stability of intermediate decides the yield of the product. Consider the attacks of H+ (an electrophile) on the propene molecule. The two inter – mediate carbocations are formed.
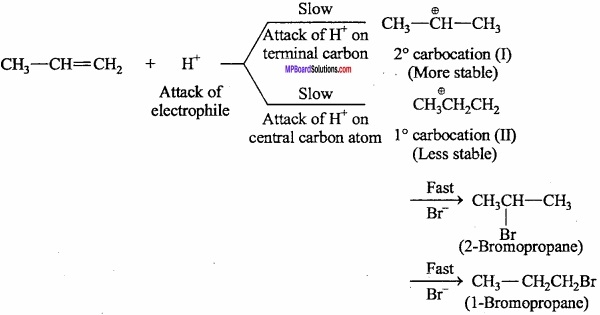
Since, a 2° carbocation (I) is more stable than 1° carbocation (II), therefore, carbocation (I) is predominantly formed. (MPBoardSolutions.com) This carbocation then rapidly undergoes nucleo – philic attack by the Br– ion forming 2 – bromopropane as the major product. Thus, MarkownikofFs addition occurs through the more stable carbocation intermediate. .
Question 15.
Draw the cis and trans structures of hex – 2 – ene? Which isomer will have higher boiling point and why?
Answer:
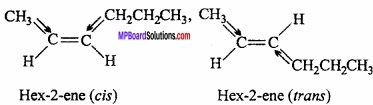
As cis isomer is more polar than trans therefore magnitude of dipole – dipole interaction is more than trans. Hence boiling point of cis isomer is more than trans.
Question 16.
An alkene, ‘A’ on ozonolysis gives a mixture of ethanal and pentan – 3 – one. Write structure and IUPAC name of ‘A’? Write structure and IUPAC name of ‘A’?
Answer:
The product of ozonolysis are –

Remove the oxygen atom (= 0) and join the two ends by a double bond, the structure of the alkene ‘A’ is

Question 17.
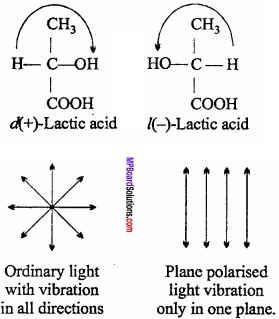
Explain Racemic mixture with example?
Answer:
The mixture of equal amount of two optical isomers is called Racemic mixture. The rotation of these two isomers are in opposite directions. So it is represented by dl or ±.
Example:
Optical isomerism or Enantiomerism:
This type of isomerism is shown by unsymmetrical compounds. Structural, physical and chemical properties of optical isomers are nearly similar but optical properties are different.(MPBoardSolutions.com) Isomer which rotates plane of polarised light in clockwise direction is called dextrorotatory and one which rotates in anti – clockwise direction is called laevo – rotatory. These two forms are represented as d – and l – or (+) and (-) respectively.
Optical isomers have unsymmetrical i.e., chiral carbon in the molecule.
Question 18.
What is B.H.C.? What is its uses?
Answer:
It is prepared by the chlorination of benzene in the presence of ultraviolet light.
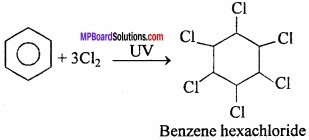
Benzene hexachloride is an addition compound and its y isomer is called Gammaxene. It is an important pesticide. It is also called Lindane or 666.
Question 19.
How will you do the following conversion?
- Methane to Ethane
- Ethane to Methane
- Acetylene to Benzene.
Answer:
1. Methane to Ethane:
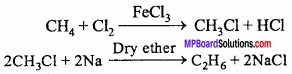
2. Ethane to Methane:
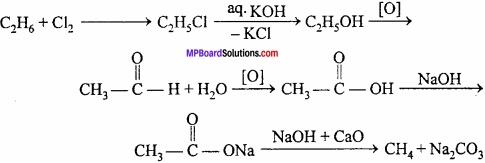
3. Acetylene to Benzene:
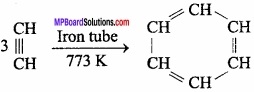
Question 20.
Write the name of the organic compounds which show geometrical isomerism in cyclic compounds?
Answer:
Some of the cyclic compounds also show geometrical isomerism.
Example:
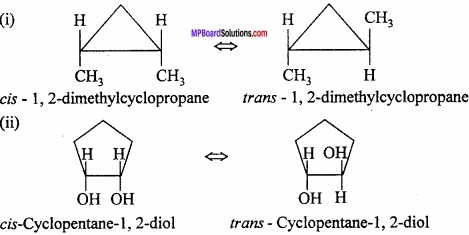
Question 21.
What is Saytzeff’s rule? Explain with example?
Answer:
Saytzeff’s rule:
According to this:
“If an alkyl halide can eliminate the hydrogen in two different ways, that alkene will be formed in excess in which carbon atoms joined by double bond are more alkylated.
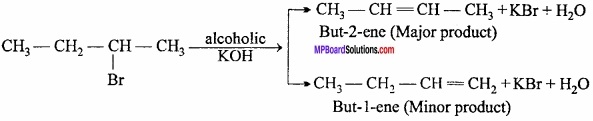
Question 22.
What are the essential conditions for a compound to show aromaticity?
Answer:
The property which gives extra stability to benzene and benzene like compounds is called aromaticity.
Aromaticity is decided by Huckel’s rule. According to Huckel’s rule, a compound will be aromatic if it fulfills the following four conditions:
- Compound shall be cyclic.
- Compound should be planar or nearly planar (sp2 hybridisation)
- Compound should be conjugated.
- Compound should have (4n + 2) π electrons. Where n is a whole number and it may be n = 0, 1, 2, 3,4, 5, 6, …
![]()
Question 23.
What is cis and trans isomerism? Explain with example?
Answer:
cis and trans isomerism is also called geometrical isomerism. This isomerism is shown by that compounds which have carbon atoms attached to two different atoms. When the two same groups or hydrogen atoms are present at one side of double bond then the compound is called cis isomer. (MPBoardSolutions.com) But when the groups or H – atoms are present in opposite side of the C double bond then such isomers are called trans isomers.
Example:
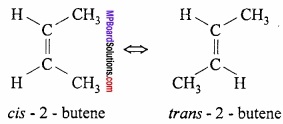
Question 24.
Arrange benzene, n – hexane and ethyne in decreasing order of their acidic behaviour? What is the reason for this behaviour?
Answer:
The hybridised state of C in the given compounds are:
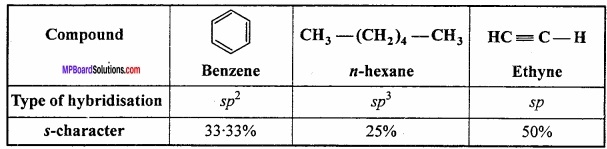
The acidic character increases with increase of s – character. So the decreasing order of acidty is:
Ethyne > Benzene > n – hexane.
Question 25.
How would you convert the following compounds into benzene:
- Ethyne
- Ethene
- Hexane.
Answer:
Ethyne:

Ethene:
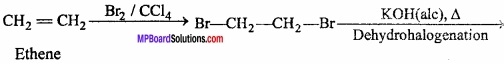
Hexane:
Question 26.
Explain why the following systems are not aromatic?
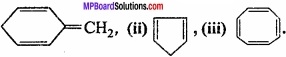
Answer:
(i)
 = CH2 does not have six electrons i.e., (4n + 2) π – electrons in the ring. Therefore, it is not an aromatic compound.
= CH2 does not have six electrons i.e., (4n + 2) π – electrons in the ring. Therefore, it is not an aromatic compound.
(ii)
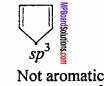
Reason: Due to sp3 hybridisation the molecule is not planar. It contains 4π – electrons, So, molecule is not aromatic.
(iii)
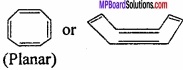
It is a conjugated system but does not have (4n + 2) π – electrons.
Hydrocarbons Long Answer Type Questions – I
Question 1.
Explain the conformation of n – butane?
Answer:
Conformations of n – butane:
n – Butane can be considered as dimethyl derivative ethane which is produced when terminal hydrogen of each carbon is replaced by methyl group. To assign conformations to n – butane is a difficult task because it has three carbon – carbon single bonds (one in the middle and two at the ends) which undergo free rotation.
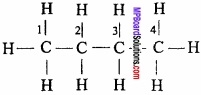
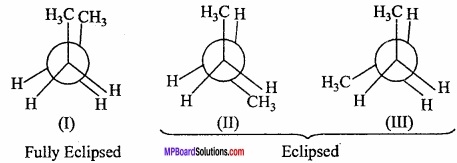
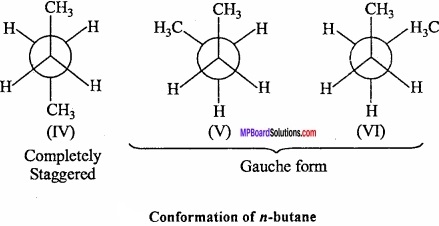
Various conformations of n – butane can be obtained by rotating C2 or C3 through 360° in six steps (60° each time). Staggered and eclipsed forms are obtained alternately on rotating C2 or C3 by 60°. These conformations are shown below. (MPBoardSolutions.com) Fully eclipsed form is shown in (I) and other eclipsed forms are shown in II and III. On rotation of C2 – C3 bond by 120°. The completely staggered form is shown in (IV). It is called Antiform also. Other staggered forms shown in V and VI are called skew or gauche forms. The staggered form IV and gauche forms V and VI are termed conformational diastereoisomers.
Question 2.
Give any four points in favour of Kekule’s formula to show its resonance structures?
Answer:
Factors in favour of Kekule’s structure:
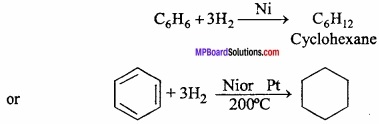
1. Benzene reacts with three molecules of hydrogen to form cyclohexane which proves presence of three double bonds.
2. Benzene reacts with three molecules of chlorine to form benzene hexachloride.
![]()
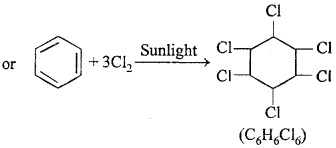
3. Three molecules of acetylene polymerize in a red hot tube to form benzene.
3CH ≡ CH → C6H6
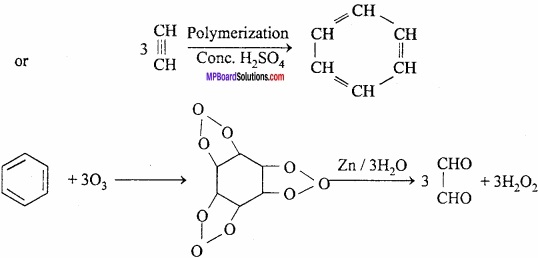
4. Oxidation of benzene with air in presence of V2O5 gives maleic acid which loses a molecule of water to form maleic anhydride.
Question 3.
A hydrocarbon ‘A’ vapour density is 14, makes the Baeyer’s reagent colourless and perform following reaction:
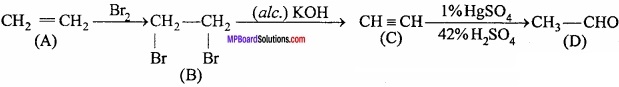
Write the name and formula of A, B, C and D?
Answer:
![]()
(A) → Ethylene
(B) → 1, 2 – dibromoethane or ethylene bromide
(C) → Acetylene
(D) → Acetaldehyde.
Question 4.
Explain the laboratory method of formation of alkene with figure?
Answer:
Laboratory preparation of alkene (ethylene):
Ethylene is obtained in laboratory by heating ethyl alcohol with excess of cone. H2SO4 at 170°C.
1. Requirements:
Ethyl alcohol, cone. H2SO4, sand bath. Thistle funnel, potassiumm hydroxide, gas jar, stand etc.
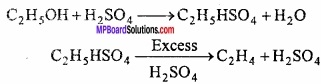
2. Chemical equation:
C2H5OH + H2SO4 → C2H5HSO4 + H2O
3. Method:
50 cc of ethyl alcohol and 100 cc of conc.H2SO4 is taken in a flask, then 8 gm anhydrous Al2 (SO4)3 and 50 gm of sand is added. The mixture of Al2 (SO4)3 and sand checks up formation of foam and facilitates the reaction to take place at 140° C.
Now the flask is fitted with a thermometer, an exit tube and a dropping funnel. Flask is placed on a sand bath and fixed with a stand. The other end of exit tube dips in wash bottle containing NaOH. Another tube from wash bottle leads to a behive shelf placed in a trough of water. (MPBoardSolutions.com) A water jar is Inverted over behive shelf. Flask is heated at a temperature of 150° C and simultaneously mixture of alcohol and cone. H2SO4 is added dropwise into the flask. Along with ethylene, CO2 (by oxidation of alcohol) and SO2 (by reduction of H2SO4) are also present as impurities in flask. These impurities get adsorbed in NaOH solution and pure ethylene is collected in gas jars by downward displacement of water. Ethylene prepared by this method is pure.
4. Labelled diagram:
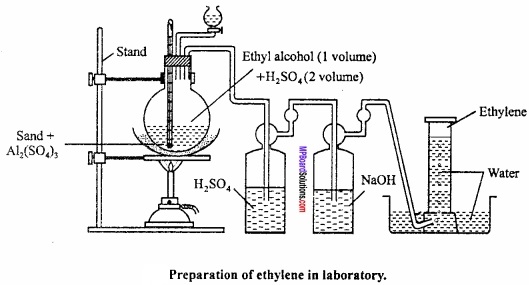
Question 5.
Explain the Laboratory method of preparation of acetylene? Or, Explain the Laboratory method of preparation of acetylene on following points:
- Method and Chemical reaction
- Labelled figure.
Answer:
Preparation of acetylene in laboratory:
Acetylene is prepared by dropping water on calcium carbide. Acetylene obtained by this method contains impurities of PH3 and NH3. These are removed by passing the gas through CuSO4 solution.
![]()
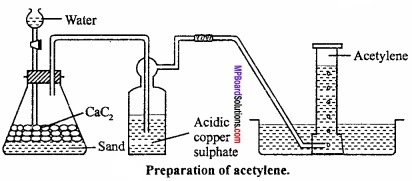
Precautions:
Before experiment, air in flask is replaced by oil gas because acetylene forms explosive mixture with air.
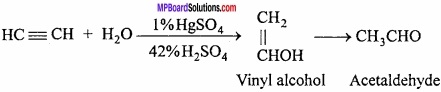
Reaction of acetylene with water:
In presence of 1% HgSO4 and 42% H2SO4, acetaldehyde is formed.
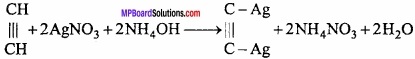
Reaction with ammoniacal silver nitrate solution:
When acetylene is passed through ammoniacal AgNO3 solution, white precipitate of silver acetylide is obtained.
Question 6.
Out of benzene, m – dinitrobenzene and toluene which will undergo nitration most easily, why?
Answer:
CH3 group is electron releasing while NO2 group is electron withdrawing. Therefore, maximum electron density will be in Toluene followed by in benzene and least in m – nitrobenzene. Therefore, the ease of nitration decreases in the order:
Toluene > Benzene > m – dinitrobenzene.
Question 7.
How will you obtain:
- B.H.C. from benzene
- Acetophenone from benzene
- P.V.C. from chloroethene
- Teflon from tetrafluoroethene.
Answer:
1. B.H.C. from benzene:
![]()
2. Acetophenone from benzene:
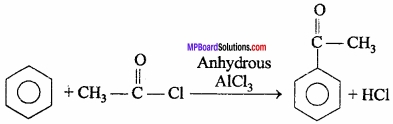
3. P.V.C from chloroethene:
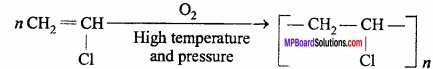
4. Teflon from tetrafluoroethene:
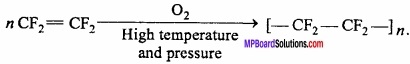
Question 8.
Write notes on following:
- Sabatier and Senderens reaction
- Wurtz reaction
- Duma’s reaction,
- Swart reaction.
Answer:
1. Sabatier and Senderens reaction:
The reaction of alkene with hydrogen in presence of Ni or Pt, after hydrogenation alkane is obtained.

2. Wurtz reaction:
Reaction of two molecules of alkyl halide with sodium in presence of dry ether, alkane is obtained.
![]()
3. Duma’s reaction:
Reaction of sodium salt of monocarboxylic acid with soda lime, after decarboxylation alkanes are formed.
![]()
4. Swart reaction:
Reaction of alkyl halide with mercuric chloride, chloroalkanes are obtained. During this reaction the substitution of halogen of alkyl halide occur by Cl.
2C2H5 – I + HgF2 → 2C2H5 – F + HgI2
Question 9.
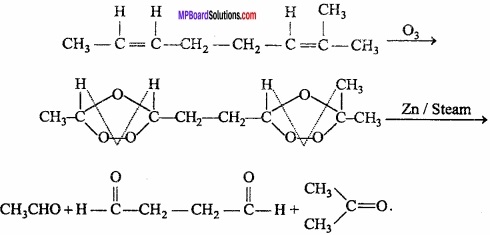
An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane – 1, 4 – dial, ethanal and propanone. Give the structure of ‘A’. Write its IUPAC name and explain the reaction involved?
Answer:
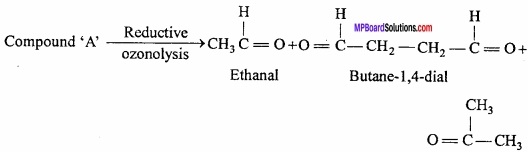
Thus, the structure of compound ‘A’ may be written as:
Hydrocarbons Long Answer Type Questions – II
Question 1.
Write the equations for the following reactions:
- Reaction of calcium carbide with water.
- Reaction of bromine water on ethylene.
- Heating of ethylene with alkaline KMnO4.
- Heating benzene with cone. HNO3 and cone. H2SO4.
- Heating benzene with methyl chloride in presence of anhydrous AlCl3
Answer:
1. Reaction of calcium carbide with water:
CaC2 + 2H.OH → CH = CH + Ca(OH)2
2. Reaction of ethylene with bromine water:

3. Heating ethylene with alkaline KMnO4:
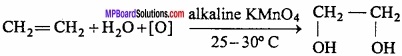
4. Heating benzene with cone. HNO3 and cone. H2SO4:

5. Heating benzene with methyl chloride in presence of anhydrous AlCl3:

Question 2.
How will you obtain following:
- Acetaldehyde from Acetylene.
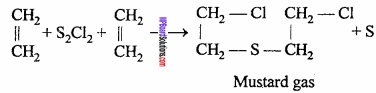
- Mustard gas from Ethylene.
- Ethane from Grignard reagent
- Cuprous acetylide from Acetylene.
- Methane from Aluminium carbide.
Answer:
1. Acetaldehyde from Acetylene:

2. Mustard gas from Ethylene:
3. Ethane from Grignard reagent:

4. Cuprous Acetylide from Acetylene:
CH ≡ CH + Cu2Cl2 +2NH4OH → Cu – C ≡ C – Cu + 2NH4Cl + 2H2O
5. Methane from Aluminium carbide:
Al4C3 + 12H2O → 3CH4 + 4Al(OH)3.
![]()
Question 3.
What is conformation? Describe conformation found in ethane?
Answer:
In alkane the C – C bond is formed by the axial overlapping of sp3 hybrid orbitals of the adjacent carbon atoms. The electron distribution in molecular orbitals of sp3 – sp3 sigma bond is cylindrically symmetrical around the inter nuclear axis. This symmetry permits the free rotation about the bond axis without rupture of the molecule. (MPBoardSolutions.com) This result in a large number of different spatial arrangement of atom or group attached to the carbon atom. Thus, “The different spatial arrangement obtained by the free rotation around the bond axis of a C – C cr bond are called conformers and the molecular geometry corresponding to a conformer is known as conformation.
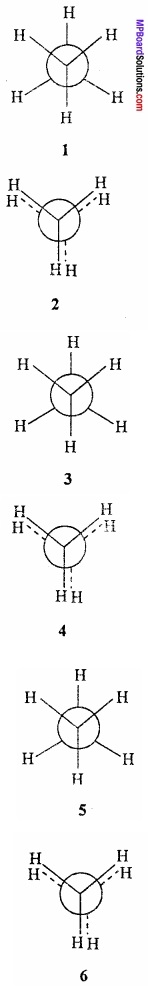
Conformation of ethane:
If the position of one carbon atom of ethane is fixed in space and the other carbon atom is rotated around the C – C bond, then various conformations of ethane are possible. Out of these the conformers which has the lowest energy is called staggered and the one having highest energy is called eclipsed conformation.
1. Staggered conformation:
In staggered conformation, the hydrogen atom of the two carbon atoms are oriented in such a way that they lie far apart from one another. In other words, they are staggered away with respect to one another.
2. Eclipsed conformation:
In eclipsed conformation, the hydrogen atoms of one carbon are lying directly behind the hydrogen atoms of the other. In other words, hydrogen atoms of one carbon are eclipsing the hydrogen atoms of the other.(MPBoardSolutions.com) The conformations of ethane do not have same stability. The staggered conformation is relatively more stable than the other conformation. The difference in the energy content of staggered and eclipsed conformation is 12.5 kJ mol-1.
Question 4.
An alkane C8H18 is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination this alkane yields a single isomer of a tertiary bromide. Write the structure of alkane and tertiary bromide?
Answer:
From Wurtz reaction of an alkyl halide gives an alkane with double the number of carbon atoms present in the alkyl halide. Here, Wurtz reaction of a primary alkyl gives an alkene (C8H16), therefore, the alkyl halide must contain four carbon atoms. Now the two possible primaiy alkyl halides having four carbon atoms each are, I and II.

Since, alkane C8H18 on monobromination yields a single isomer of tertiary alkyl bromide, therefore, the alkene must contain tertiary hydrogen. This is possible, only if primary alkyl halide (which undergoes Wurtz reaction) has a tertiary hydrogen.
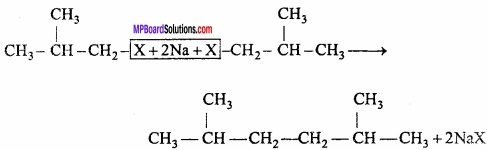
Question 5.
Explain the free radical mechanism of halogenation of alkane?
Answer:
Mechanism of halogenation of alkane:
Halogenation of alkanes proceed through the formation of free radicals. Therefore, it is also called free radical substitution. The reaction proceeds in the following steps:
1. Chain initiation step:
The first step involves the homolytic fission of chlorine molecule to form chlorine free radicals. This fission takes place in the presence of light or heat.

Note that Cl – Cl bond being weaker than C – H bond and the C – C bonds. Therefore, undergo cleavage first.
2. Chain propagation:
Chlorine free radical is produced in the first step, attacks methane molecule forming methyl free radical and HCl. Methyl free radical reacts with other chlorine molecule forming methyl chloride and chlorine free radical.
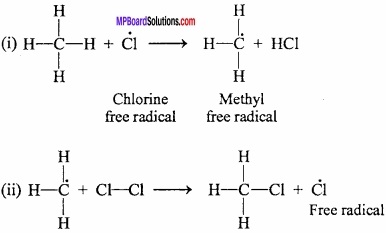
When sufficient amount of methyl chloride has been formed, the Cl produced in reaction (ii) has a greater chance of colliding with a molecule of CH3Cl rather than a molecule of CH4. If such a collision occurs, a new free radical (CH2Cl) is produced.
CH3Cl + \(\overset { \bullet }{ C } \) l → CH2Cl + HCl
This \(\overset { \bullet }{ C } \)H2 Cl then reacts with Cl2 to give CH2Cl2 and another \(\overset { \bullet }{ C } \) l free radical.
\(\overset { \bullet }{ C } \)H2Cl + Cl2 → CH2Cl2 + \(\overset { \bullet }{ C } \) l
This process continues till all the hydrogen is removed.
3. Chain termination:
The chain reaction steps if two or different free radicals combine amongst themselves without producing new free radicals. The possible termination steps are as follows:
\(\overset { \bullet }{ C } \) l + \(\overset { \bullet }{ C } \) l → Cl2
\(\overset { \bullet }{ C } \) H3 + \(\overset { \bullet }{ C } \) H3 → CH3 – CH3
\(\overset { \bullet }{ C } \) H3 + \(\overset { \bullet }{ C } \) l → CH3Cl
Question 6.
Explain the conformational isomerism in cyclohexane?
Answer:
Conformations of Cyclohexane:
Like alkanes cyclohexane (a cycloalkane) also exhibits conformational isomerism. Sachse (1890) suggested that if cyclohexane has a planar cyclic hexagonal structure, then it will be highly stable due to the presence of angular strain of the ring composed of sp2 hybrid carbon atoms. (MPBoardSolutions.com) However, cyclohexane is quite stable. Sachse suggested that two non – planar models are possible which are free from angular strain. These are called chair and boat conformations as shown in Fig.
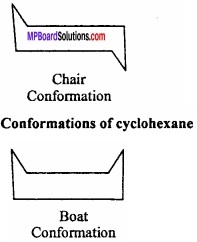
These are in a staggered way and eclipsed conformation of alkanes. The chain form is more stable that the boat form by an energy equal to about 7 kcal/mol. In the boat form, like the eclipsed form of ethane, the hydrogen atoms being very close repel each other and the system becomes unstable. (MPBoardSolutions.com) In the boat form there is considerable non – bonded interaction between the flagpole hydrogens and also between other eclipsed hydrogens. As a consequence, this form of cyclohexane can flex into what is known as twist boat form (flexible form) which is stable by about 1.8 kcal/mol than regular boat.
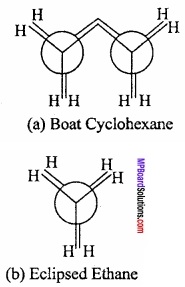
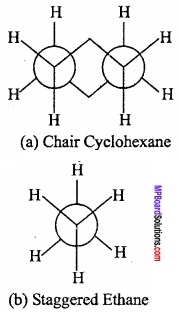
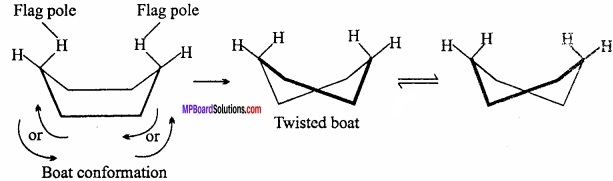
In the chain form, the hydrogen atoms are situated quite far apart from one another. As a result the force of repulsion between the nearest (v icinal) hydrogen atoms is minimum. Thus, out of two main conformations, chair and boat, the chair form is more stable and cyclohexane exists mainly in chair form.
Question 7.
Explain the electrophilic substitution reactions in aromatic hydrocarbons giving two examples?
Answer:
Mechanism of monosubstitution in benzene:
Study of many monosubstitution reactions of benzene show that these reactions follow mechanism of electrophilic substitution. In thesi 40% H2SO4tie reagent is an electrophile (E+). It can be understood in the following steps:
Step I.
Generation of electrophile:
Dissociation of attacking reagent results in the formation of electrophile (E+).
E – Nu → E+ + Nu–
Step II.
Attack of the electrophile on the ring:
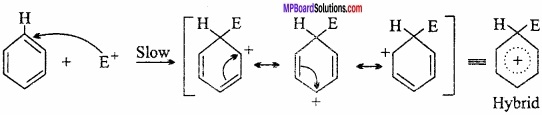
Step III.
Abstraction of a proton by the base:
Nucleophile displaces proton from the hybrid in fast step forming desired substituted products. In this way elimination takes place in this step.
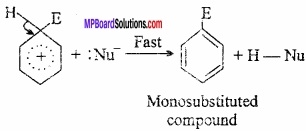
In this way in electrophilic substitution reaction of benzene, electrophile is added in first step and then H+ ion is eliminated.
Example 1.
Mechanism of nitration of benzene:
Nitration of benzene is done in the ahead steps by treating with a nitrating mixture of concentrated nitric acid and concentrated sulphuric acid:
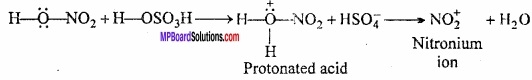
Step I.
Generation of electrophile (NO2+):
Step II.
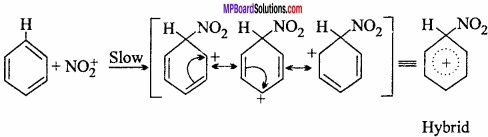
Attack of the electrophile on the ring:
Electrophile attacks on the benzene ring forming carbocation. It attains stability by ion resonance.
Step III.
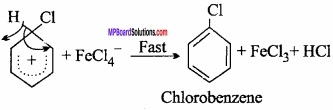
Abstraction of H+ ion by the base:
Nucleophile HSO4– ion substitutes H+ ion of the ring in fast step forming nitrobenzene.
Example 2.
Mechanism of halogenation of benzene:
Mechanism ofhalogenation of benzene can be explained by the action of chlorine on benzene in presence of Lewis acid FeCl3.
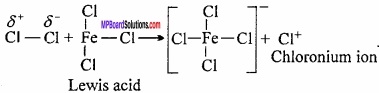
Step I.
Generation of electrophile (Cl+):
Step II.
Attack of the electrophile on the ring:
Electrophile attacks on the ring forming carbocation intermediate which is resonance stabilised.
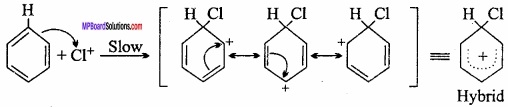
Step III.
Abstraction of H+ ion by the base:
FeCl4– ion is a base here and it displaces H+ ion from hybrid forming desired products in the fast step.
