In this article, we will share MP Board Class 11th Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure Pdf, These solutions are solved by subject experts from the latest edition books.
MP Board Class 11th Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
MP Board Class 11 Chemistry Chemical Bonding and Molecular Structure Textbook Questions and Answers
Question 1.
Explain the formation of chemical bond. –
Solution.
According to Kossel-Lewis approach, the formation of chemical bond between the two atoms takes place either by the transference of electrons or by mutual sharing of electrons. However, according to the modern view the formation of chemical bond between the two approaching atoms occurs only if there is a net decrease of energy because of attractive and repulsive forces.
Question 2.
Write the Lewis dot symbols of the following elements: Mg, Na, B, O, N, Br.
Solution.
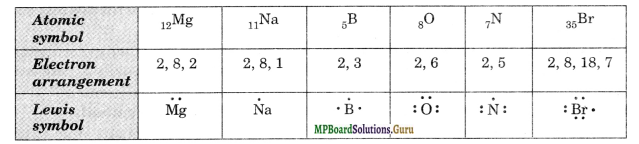
Question 3.
Write the Lewis dot symbols of the following atoms and ions: S and S2- ; Al and Al3+ ; H and H–
Solution.
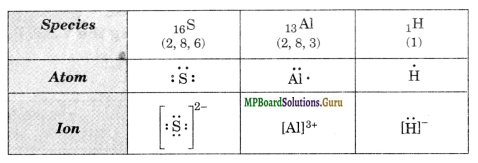
Question 4.
Draw the Lewis structures of following molecules and ions H2S, SiCl4, BeF2, COHCOOH.
Solution.
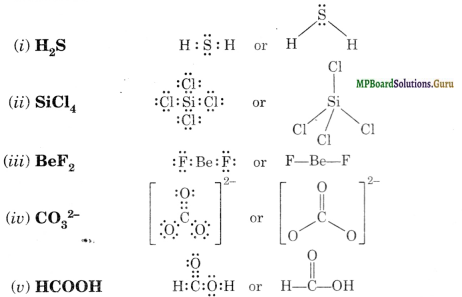
Question 5.
Delne Ocetet rule. Write its significance and limitations.
Solution.
Octet Rule: The tendancy of atoms to achieve eight electrons in their outer¬most shell is known as Lewis octet rule.
Limitations of the Octet Rule:
(i) The incomplete octet of the central atom (electron deficient compound )

(ii) Formation of compounds involving hydrogen: Hydrogen atom contains one elec¬tron, it needs one more electron to complete its shell to acquire the nearest noble gas configuration.
(iii) Odd electron bonds/Odd electron molecules: There are some molecules and ions in which the bonded atoms contain odd number of electrons (usually 3) between them.
For examples: NO, NO2.
![]()
Question 6.
Write the favourable conditions for the formation of ionic bond.
Solution.
Ionic bond is formed by transference of electrons from one atom to another. ;
The favourable conditions for its formation are :
- Low ionisation enthalpy of element forming cation.
- More negative value of electron gain enthalpy of element forming the anion and
- High value of lattice enthalpy of the compound formed.
Question 7.
Predict the shapes of the following molecules using the V.S.E.P.R. model.
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Solution.
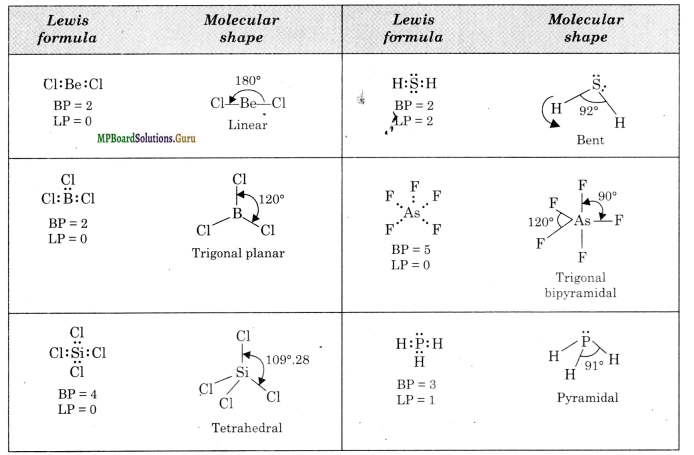
Question 8.
Although geometries of NH3 and HaO molecules are distorted tetrahedral, bond angle in water is less than that in ammonia. Discuss.
Solution.
The difference in bond angles is due to the different numbers of lone pairs and bond pairs in the two species. In NH3, the N atom has one lone pair and three bond pairs while in H2O, the O atom has two lone pairs and two bond pairs. The repulsive interactions of lone pairs and bond pairs in water are relatively more than those in NH3. Hence, bond angle around central atom in water is relatively smaller than that in NH3 molecule.
Question 9.
How do we express bond strength in terms of bond order?
Solution.
Bond strength is directly related to bond order. This means that larger the bond order, more is the bond strength.
Question 10.
Define bond length.
Solution.
The equilibrium distance between the centres of the nuclei of the two bonded atoms is called its bond length.
Question 11.
Explain the important aspects of resonance with respect to the CO23 ion.
Solution.
In case of certain molecules, a single Lewis structure cannot explain all the properties of the molecule. The molecule is then supposed to have many structures, each of which can explain most of the properties of the molecule but none can explain all the properties of the molecule. The actual structure is in between of all these contributing structures and is called resonance hybrid and the different individual struc¬tures are called resonating structures or canonical forms. This phenomenon is called resonance. For example, the structure of carbonate ion CO2-3 can be written as
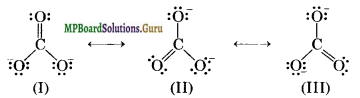
Question 12.
H3PO3 can be represented by the structures 1 and 2 as shown below. Can these structures be taken as the canonical forms of the resonance hybrid representing H3PO3 ? If not cite reasons for the same.

Solution.
These structures cannot be taken as the canonical forms. As per the requirement of resonance, the canonical forms differ only in electronic arrangements but they should have same atomic arrangement.
![]()
Question 13.
Write the resonating structures for SO3, NO2 and NO3–.
Solution.
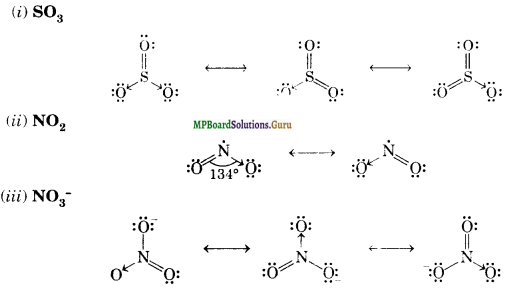
Question 14.
Using Lewis dot symbols, show electron transfer between the following atoms to form cations and anions:
(a) K and S
(b) Ca and O
(c) Al and N.
Solution.
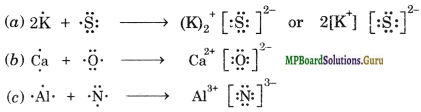
Question 15.
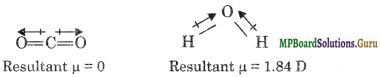
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Solution.
The bond moments of two C = O bonds in CO2 cancel each other indicating the linear structure for CO2. The resultant µ for H2O molecule is 0. This indicates a bent structure for H2O molecule because bond moments of two O – H bonds do not cancel out.
Question 16.
Write the significance and applications of dipole moment.
Solution.
Significance of dipole moment: Dipole moment can be determined experimentally and its value can give an idea of the polar character of a molecule. It is a vector quantity as it has a direction as well as magnitude. The direction of dipole moment is usually represented by an arrow ( →) pointing from positive end towards the negative end.
Question 17.
Define electronegativity. How does it differ from electron gain enthalpy?
Solution.
Electronegativity is the ability of an atom to attract the bond pair or shared pair of electrons towards itself in a molecule. On the other hand, electron gain enthalpy is the energy released when one mol of gaseous atoms, of the element accepts an electron to form gaseous uni-negative ion.
Question 18.
Explain with the help of suitable examples, the term polar covalent bond.
Solution.
Polar covalent bonds: A covalent bond develops a partial ionic character as a result of the difference of electronegativities of the atoms comprising the bond.
In the other word, when two atoms forming a covalent bond are dissimilar their electronegativities are different, the pair forming the bond will be closer to the more electronegative atom and as a result such atom will acquire a partial -ve charge (denoted by δ-) and the other atom a partial +ve charge (δ+). For example, (i) HCl (ii) H2O
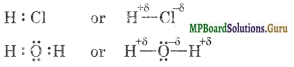
Question 19.
Arrange the bonds in the order of increasing ionic character in the molecules:
LiF, K2O, N2, SO2 and ClF3
Solution.
The ionic character of the molecule depends upon the difference of electronegativities of the atoms involved in bond formation and also the geometrical arrangement of the bonds. Among the given species the correct arrangement is N2 < SO2 < ClF3 < K2O < LiF.
![]()
Question 20.
The skeletal structure of CH3COOH as shown below is correct but some of the bonds are wrongly shown. Write the correct Lewis structure of acetic acid.
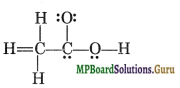
Solution.
The skeletal arrangement of the atoms in the above structure is correct, however, electronic arrangement is not in accordance with Lewis concept. The correct structure of acetic acid is
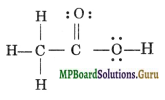
Question 21.
Apart from tetrahedral geometry for methane (CH4), another possible geometry is square planar with four ‘H’ atoms at the corners of the square with the ‘C’ atom at its centre. Explain why CH4 is not square planar.
Solution.
The tetrahedral and square planar structures of CH4 are shown :
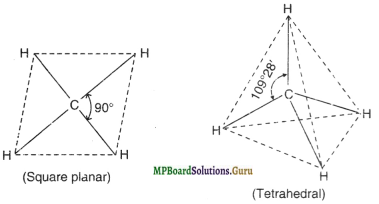
According to VSEPR theory, the electron pairs around the central atom lie as far apart as possible so as to minimise the repulsive interactions between them. For square planar arrangement for CH4, the four C-H bond pairs have to lie at an angle of 90°.
But in tetrahedral arrangement, the bond pairs of C-H bonds lie at an angle of 109°28′ and therefore, have smaller repulsive interactions than those in square planar arrangement.
Question 22.
Explain why BeH2 molecule has zero dipole moment although the Be-H bonds are polar.
Solution.
BeH2 is a linear molecule with (H-Be-H) bond angle equal to 180°. Although the Be-H bonds are polar on account of electronegativity difference between Be and H atoms, the bond polarities cancel with each other. The molecule has resultant dipole moment zero.
Question 23.
Which out of NH3 and NF3 has higher dipole moment and why?
Solution.
µ (NH3) = 1.46D which is greater than µ(NF3) = 0.25D.
In NH3 the bond moments of three N-H bonds reinforce the lone pair moment while in NF3 the three N-F bond moments oppose the lone pair moment.
![]()
Question 24.
What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2 and sp3 hybrid orbits.
Solution.
Hybridisation: Intermixing of different orbitals having slightly different energies, achieve the identical shape and equal energies are called hybridisation.
Shape of sp, sp2 and sp3 hybrid orbits
sp = linear
sp2 = trigonal
sp3 = Tetrahedral
(For more details see section 4.6)
Question 25.
Describe the change in hybridisation (if any) of the A1 atom in the reaction
AICl3 + Cl- → AlCl4
Solution.
In AICl3, X = 3 + \(\frac{1}{2}\) (3-3) = 3
∴ Hybrid state of Al= sp2
In [AlCl4]-, X = 4+ \(\frac{1}{2}\)(3-3+1) = 4
∴ Hybrid state of Al = sp3.
Question 26.
Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
BF3 + NH3 → F3B : NH3
Solution.
During combination of species BF3 and NH3, N atom is donor and B atom of BF3 is acceptor. The hybrid state of B in BF3 is sp2 and that of N in NH3 is sp3. In the compound F3B+-NH3 both N and B atoms are surrounded by four bond pairs. Thus, the hybrid state of both is sp3. Hence during the reaction the hybrid state of B changes from sp2 to sp3 but that of N remains the same.
Question 27.
Draw diagrams showing the formation of a double and a triple bond between the carbon atoms in the C2H4 and C2H2 molecules.
Solution.
Formation of ethylene (C2H4): Both the carbon atoms in ethylene assume sp2 hybrid state. In acquiring sp2 hybrid state, one 2s-orbital and two 2p-orbitals of excited carbon atom get hybridised to form three sp2 hybridised orbitals. However, one orbital of 2p-subshell of the excited carbon atom does not take part in hybridisation.
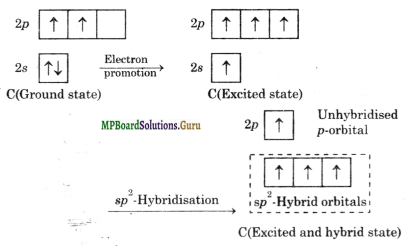
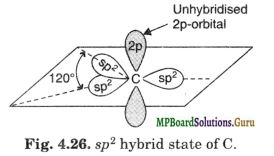
As already indicated, the three sp2 hybrid orbitals lie in one plane and are oriented in space at an angle of 120° to one another. The unhybridised 2p-orbital is perpendicular to the plane of sp2 hybrid orbitals.
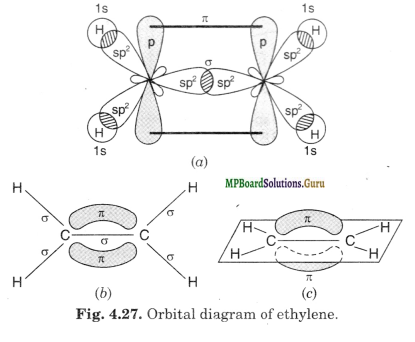
In the formation of ethylene (C2H4), one of the sp2 hybrid orbital of carbon atom over¬laps axially with similar orbital of the other carbon atom to form C-C sigma bond. The other two sp2 hybrid orbitals of each carbon atom are utilised for forming sp2-s sigma bond with two hydrogen atoms.
The unhybridised 2p-orbitals of the two carbon atoms overlap sidewise each other to form two n clouds distributed above and below the plane of carbon and hydrogen atoms.
Thus, in ethylene, the six atoms (bonded by sigma bonds) lie in one plane while the n bond is projected perpendicular to the plane of six atoms (two C atoms and four H atoms).
In ethylene molecule, the C = C bond consists of one sp2-sp2 sigma bond and one it bond. Its bond length is 134 pm. C-H bond is sp2-s sigma bond with bond length 108 pm. The H-C-H angle is 117.5° while H-C-C angle is 121°.
Formation of acetylene (CH≡CH). Both the carbon atoms in acetylene assume sp-hybrid state. In sp-hybrid state, one 2s orbital and one 2p-orbital of excited carbon atom (1s2 2s2 2px1 2py1 2pz1) get hybridised to form two sp-hybridised orbitals.
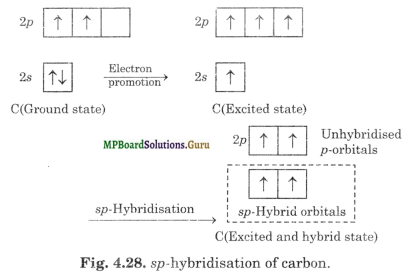
The two sp-hybrid orbitals of carbon atom are linear and are directed at an angle of 180° whereas the unhybridised p-orbitals are perpendicular to sp-hybrid orbitals and also perpendicular to each other as shown in Fig. 4.29
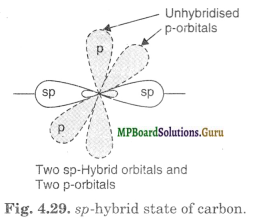
In the formation of acetylene, carbon atom uses its one of the sp-hybrid orbital for overlapping with similar orbital of the other carbon to form C-C sigma bond. The other sp-hybrid orbital of each C atom overlaps axially with Is-orbital of H atom to form C-H sigma bond. Each of the two unhybridised orbitals of both the carbon atoms overlap sidewise to form two rc-bonds. The electron clouds of one Tc-bond lie above and below the inter-nuclear axis whereas those of the other n bond lie in front and back of the inter-nuclear axis.
The overlapping of orbitals has been shown in Fig. 4.30
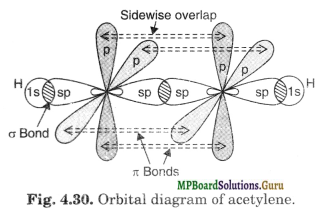
The four n clouds so formed further merge into one another to form a single cylindrical electron cloud around the inter-nuclear axis representing C-C sigma bond. It has been shown in Fig. 4.31.
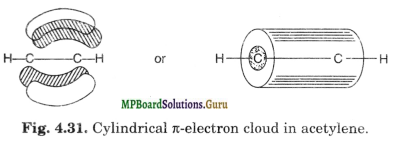
Thus in acetylene molecules C≡C bond consists of one sp-sp a bond along with two n-bonds. The C≡C bond length is 120 pm. C-H bond is sp-s sigma bond. The H-C-C angle is 180°, that is molecule is linear.
![]()
Question 28.
What is the total number of sigma and pi bonds in the following molecules?
(a) C2H2
(b) C2H4

Question 29.
Considering x-axis as the inter-nuclear axis, which out of the following will form a sigma bond?
(a) 1s and 1s
(b) 1s and 2px
(c) 2py and 2py
(d) Is and 2s.
Solution.
The sigma bond is formed by axial overlap and it can be formed by following orbitals.
(a) 1s and 1s
(b) 1s and 2px
(c) 1s and 2s.
Question 30.
Which hybrid orbitals are used by the carbon atoms in the following molecules?
(a) H3C-CH3
(b) H3C-CH – CH2
(c) CH3-CH2OH
(d) CH3CHO
(e) CH3COOH.
Solution:

Question 31.
What do you understand by bond pairs and lone pairs of electrons. Illustrate by giving one example of each type.
Solution.
The pair of electrons which are shared by the two atoms to constitute a covalent bond is called bond pair. On the other hand the electron pair of the valence shell which is not used in sharing/bonding is called lone pair. For example, in CH4 there are only four bond pairs; in NH3 molecule, there are 3 bond pairs, and one lone pair and in water molecule there are two lone pairs and 2 bond pairs as shown below.
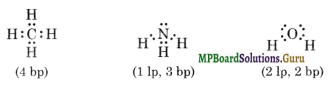
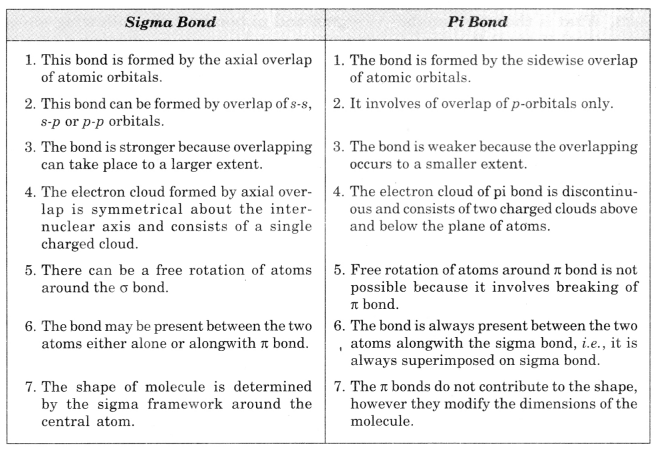
Question 32.
Differences between a sigma and a pi bond.
Solution.
Question 33.
Explain the formation of H2 molecule on the basis of valence bond theory.
Solution.
We know that hydrogen atom has one electron which is present in its 1s-orbital. Let us assume two hydrogen atoms, HA and HB, with their corresponding electrons eA and eB respectively. When the two atoms start approaching, the electrons of each atom start coming under the influence of the nucleus of the other. In other words, their orbit¬als begin to interact. The following new electrostatic interactions are set up :
(a) Attractive forces between :
(i) electron eA and nucleus HB
(ii) electron eB and nucleus HA
(b) Repulsive forces between :
(i.) electron eA and electron eB
(ii) nucleus HA and nucleus HB
The attractive and repulsive forces have been shown in Fig. 4.32
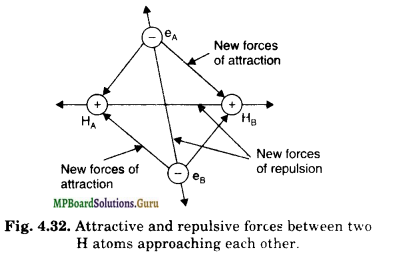
The attractive forces tend to bring the two atoms close to each other whereas repulsive forces tend to push them apart. It has been found that in the beginning the magnitude of attractive forces is greater than the repulsive forces. Therefore, the atoms go on approaching each other and the potential energy of the system continues decreasing. Ultimately, a state is reached where attractive forces, just balance the repulsive forces.
Question 34.
Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Solution.
Conditions for the linear combination of atomic orbitals:
- The combining atomic orbitals should have same energies.
- The combining atomic orbitals must have same symmetry about the molecular axis.
- The combining atomic orbitals must overlap to the maximum extent.
Question 35.
Use molecular orbital theory to explain why Be2 molecule does not exist.
Solution.
The atomic No. (Z) of Be is 4. This means that 8 electrons are to be filled in the molecular orbitals of Be2. The M.O. configuration is :
σ1s2, σ*1s2, σ2S2, σ*2S2
Bond order = \(\frac{1}{2}\) [Nb – Na] = \(\frac{1}{2}\) [4 – 4] = 0
As the bond order comes out to be zero, the molecule of Be2 does not exist.
![]()
Question 36.
Compare the relative stability of the following species and indicate their magnetic properties:
O2,O2+, O2–(superoxide), O22- (peroxide)
Solution.
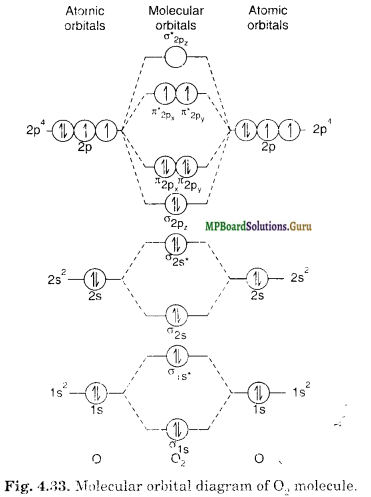
Oxygen Molecule (O2)
The electronic configuration of oxygen is 1s2, 2s2, 2p4. Each oxygen atom has 8 electrons, hence, in O2 molecule there are 16 electrons. The electronic configuration of O2 molecule, therefore, is
![]()
From the electronic configuration of O2 molecule it is clear that in it ten electrons are present in bonding molecular orbitals and six electrons are present in anti-bonding molecular orbitals. Its bond order, therefore, is
Bond order = \(\frac{1}{2}\) [Nb – Na] =\(\frac{1}{2}\) [10 – 6]=2.
So in oxygen molecule, atoms are held by a double covalent bond. Oxygen contains two unpaired electrons in π*2px1and π*2py1 molecular orbitals. Therefore, O2 molecule has paramagnetic nature.
The molecular orbital diagram of O2 molecule is shown in Fig. 4.33.
Oxygen Molecule Ion (O+2)
This ion is formed by removal of one electron from O2 molecule.
O2 – e– → O2+
From the molecular orbital diagram (Fig. 4.33) of O2 (given on previous page) it is clear that this electron would be removed from one of the anti-bonding molecular orbitals
(π*2px or π*2Py ). Therefore, the electronic configuration of O2+ ion is
![]()
and its bond order is = \(\frac{10-5}{2}\) = \(\frac{5}{2}\) = 2.5
Since bond order of O2+ is greater than the bond order of O2, therefore, it will have smaller bond length and larger bond dissociation energy than that of O2 molecule. Its bond length is 112 pm and bond dissociation energy is 625 kJ/mole. Moreover, it exhibits paramagnetic character because of the presence of an unpaired electron.
Superoxide Ion (O2–)
This ion is formed by addition of one electron to O2 molecule.
O2 + 2e– → O22-
This additional electron enters into one of the anti-bonding orbitals (π*2px π2Py*). Therefore, electronic configuration of O2– ion is
![]()
and its bond order is = \(\frac{10-7}{2}\) = \(\frac{3}{2}\) = 1\(\frac{1}{2}\) .
Since bond order of O2– is less than that of O2 molecule, therefore, it will have longer bond length and smaller bond dissociation energy than O2 molecule. It is also paramagnetic because of the presence of an unpaired electron.
Peroxide Ion (O22-)
This ion is formed by addition of two electrons to O2 molecule.
O2 + 2e– → O22-.
These additional electrons enter the two half-filled K anti-bonding orbitals. Therefore, electronic configuration of O22- ion is
![]()
and its bond order is = \(\frac{10-8}{2}\) = 1.
Since bond order of O22- ion is less than that of O2 molecule, therefore, it will have longer bond length and smaller bond dissociation energy than that of O2 molecule. Since there is no unpaired electron in it, it is diamagnetic in nature.
Bond orders of the different species are as given in brackets.
O2(2.0),O2+ (2.5), O2– (1.5), O22-(1.0)
Relative stability :
O2+ > O2 > O2– > O22-.
![]()
Question 37.
Write the significance of plus and minus signs in representing the orbitals.
Solution.
An orbital is a pictorial representation of wave function. The plus and minus signs do not represent charges but instead they represent +ve and -ve nature of the wave function ‘Ψ’.
Question 38.
Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?,
Solution.
In the formation of PCl5, phosphorus atom assumes sp3d hybrid state. The longer nature of axial bonds is due to relative stronger repulsive interactions experi¬enced by the axial bond pairs as compared to equatorial bond pairs.
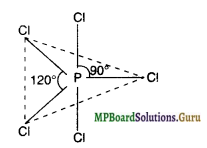
Formation of PCl5 (spad Hybridisation)
P15 = 1s2, 2s2, 2p6, 3s2, 3p3 (Ground state)
= 1s2, 2s2, 2p6, 3s1, 3p3, 3d1 (Excited state)
Now five orbitals that is one s, three p and one d orbital are available for hybridisation to yield a set of five sp3d hybrid orbitals (trigonal-bipyramidal).
Three of these hybrid orbitals are oriented towards the three corners of an equilateral triangle making an angle of 120° between them. Such bonds are called equatorial bonds. The remaining two P-Cl bonds are perpendicular to the plane of equatorial bonds, one above and one below the equitorial plane. These bonds are called axial bonds.
The axial bond pairs suffer more repulsion from the equatorial bond pairs, therefore, the axial bonds are slightly elongated and hence weaker than equatorial bonds. This makes PCl5 a highly reactive molecule.
Question 39.
Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Solution.
Hydrogen bonding: It can be defined as the partial attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O or \(\overline{\mathbf{N}}\) ) of another molecule. Hydrogen bond is represented by a dotted line (……………………) while a solid line represents a covalent bond. For example
(i) HF molecule, the hydrogen bond exists between hydrogen atom of one molecule and fluorine atom of another molecule as depicted below:
![]()
Here, hydrogen bond acts as a bridge between two atoms which holds one atom by covalent bond and the other by hydrogen bond.
(ii) H2O molecule
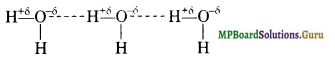
(iii) NH3 molecule
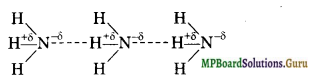
Question 40.
What is meant by the term bond order? Calculate the bond order of: N2,O2,O2– and O22-
Solution.
Bond order:
It is defined as one half the difference between the number of electrons present in bond¬ing and anti-bonding molecular orbitals.
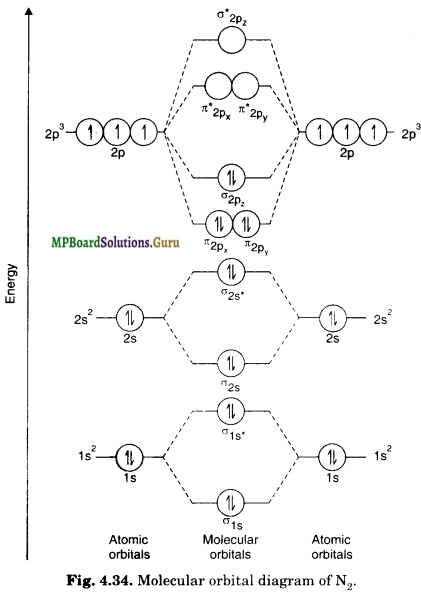
Nitrogen Molecule (N2)
Each nitrogen atom has seven electrons. Thus, N2 molecule has 14 electrons which are to be accommodated in orbitals in order of increasing energy. The molecular orbital configuration of N2 molecule is:
![]()
It is evident that there are 10 bonding electrons and 4 anti-bonding electrons. There¬fore, the bond order may be calculated as:
Bond order = \(\frac{1}{2}\) [Nb – Na] =\(\frac{1}{2}\) [10 – 4] = 3
The bond order suggests the bond energy to be very high. The experimental value of 945 kJ/mole is consistent with the fact. Since all the electrons in nitrogen are paired, it is a diamagnetic molecule.
MP Board Class 11 Chemistry Chemical Bonding and Molecular Structure Important Questions and Answers
Question 1.
Can we calculate the valency of an element from its Lewis symbol?
Answer:
Yes, the valency of an element is either equal to the number of dots or 8 minus number of dots.
Question 2.
What is conveyed by the magnitude of lattice energy?
Answer:
The magnitude of lattice energy gives an idea about the inter-ionic forces and stability of ionic solid.
Question 3.
List the factors on which lattice energy depends.
Answer:
(i.) Size of the ions
(ii) Charge on the ions.
Question 4.
What type of bonding is likely in a binary compound when both elements are ions of opposite electric charges?
Answer:
Ionic or electrovaient bond.
![]()
Question 5.
What is electrovalency?
Answer:
It is the number of electrons lost or gained by an atom during the formation of electrovaient bond.
Question 6.
What is covalency?
Answer:
It is the number of half-filled atomic orbitals which an atom provides for participation in overlapping at the time of bonding.
Question 7.
What is dative or coordinate bond?
Answer:
Dative bond or coordinate bond is the bond formed by the sharing of electrons in which shared pair of electrons is contributed by one of them called donor while the other atom simply shares it and is called acceptor.
Question 8.
What are hybrid orbitals?
Answer:
The orbitals, identical in shape and size formed by the process of hybridisation are known as hybrid orbitals.
Question 9.
Chlorine forms a more polar hydride than iodine, why?
Answer:
Because chlorine is a more electronegative element than iodine.
Question 10.
The molecule of SO2 has a dipole moment. Is the molecule linear or bent?
Answer:
Bent.
Question 11.
Which of the following molecules is superoetate?
CO2, ClF3, SO2, IF5
Answer:
ClF.j and IF-.
Question 12.
On the basis of VSEPR theory predict the shape of NH4+.
Answer:
Tetrahedral.
Question 13.
Arrange the following species in the decreasing order of their bond angle.
NH3, NH4+, NH2–
Answer:
NH3 > NH4+> NH2–.
![]()
Question 14.
Row many a are there in C2H6 (ethane)?
Answer:
7 (seven).
Question 15.
In which one is bond length C-C larger? C2H6 or C2H4.
Answer:
In C2H6.
Question 16.
BF3 is a Lewis acid or a Lewis base? Why?
Answer:
BF3 is a Lewis acid because it is an electron-deficient compound. (B has only 6 electrons)
Question 17.
In each of the following pairs select one having larger bond angle.
(i) CO2, BF3
(ii) NH3, CH4
Answer:
(i) CO2
(ii) CH4.
Question 18.
Predict the shape of the AB5 molecules.
Answer:
Trigonal pyramidal.
Question 19.
In BF3 molecule all the three B-F bonds are polar. However BF3 is a nonpolar compound. Explain.
Answer:
BF3 has a trigonal shape and the resultant of three equal dipoles at 120° gives a resultant dipole moment of zero and thus BF3 molecule is non-polar.
Question 20.
Arrange the following in increasing order of their ionic character.
LiCl, LiBr, Lil.
Answer:
Lil < LiBr < LiCl.
![]()
Question 21.
Why is there no hydrogen bonding in HCl?
Answer:
Because of large size of Cl atom.
Question 22.
What are σ electrons?
Answer:
Electrons involved in the formation of a σ bond are called σ-electrons.
Question 23.
Can π-bond be formed in the absence of a π bond?
Answer:
No.
Question 24.
What is the value of 1 Debye in Coulombs metres?
Answer:
3.34 × 10-30 Cm.
Question 25.
What type of bonds are present in ice?
Answer:
Covalent bond and hydrogen bonds.
Question 26.
Draw the Lewis dots symbols for the following elements
(i) Sodium
(ii) Calcium
(iii) Boron
(iv) Bromine.
Answer:
(i) Na
(ii) Ca
(iii) B
(iv) Br
Question 27.
Why are noble gases poor chemical reactants’.’
Answer:
The chemical inertness of the noble gases is due to the stable ns2 np6 electronic configuration in all the noble gases except Helium in the valence shell of their respective atoms. Helium too has Is2 as its fully filled stable configuration. Therefore these elements have practically no tendency to lose, gain or share any electron. Thus the noble gases are poor chemical reactants.
![]()
Question 28.
Why is only H2 molecule formed and not H3 or H4?
Answer:
H2 molecule is formed by the overlapping of 1s-orbital of each hydrogen atom (having electron of opposed spiny As no more valency orbitals and valence electrons are left so no further hydrogen can be bonded to this system and H3; H4 etc., cannot be formed.
Question 29.
Why He2 molecule does not exist?
Answer:
The valence shell of He is completely filled (1s2 configuration) and it cannot accommodate more electrons. So the valence shell orbitals of helium atom do not overlap.
Question 30.
State the geometry and bond angles for the following types of hybridisation.
(i) sp
(ii) sp2
(iii) sp3
(iv) sp3d.
Answer:
(i) sp – linear 180°
(ii) sp2 – Trigonal 120°
(iii) sp3 – Tetrahedral 109° 28′
(iv) sp3d – Trigonal bipyramidal 120° and 90°.
Question 31.
Suggest giving reasons, which substance in each pair is likely to have higher normal boiling point.
(i) HF or HCl
(ii) H2O or CH3OH
Answer:
(i) HF because of strong hydrogen bonding
![]()
(ii) H2, because of stronger hydrogen bonding, there are two O-H bonds.
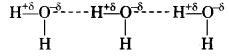
Question 32.
What is meant by the term ‘bond order’ in Lewis concept? Calculate the bond order of N2, O2 and CO.
Answer:
Bond order refers to the number of bonds between the two atoms in a molecule.
Bond order in N2 (N≡N) = 3
Bond order in O2 (0≡0) = 2
Bond order in CO (C+≡0) = 3
![]()
Question 33.
State the bond characteristics with suitable examples.
Answer:
Covalent bonds are characterised by the following parameters
- Bond enthalpy. It is the amount of energy required to break one mole of the bonds of the same kind between two atoms so as to separate the bonded atoms in the gaseous state, e.g., Cl-Cl bond enthalpy is 237 kJ mol-1 and H-H bond energy is 433 kJ mol-1.
- Bond length. It is the average distance between the centres of nuclei of the two bonded atoms in a molecule, e.g. the bond length of H-Cl bond is 127 pm and of C-Cl bond is 177 pm.
- Bond angle. It is the average angle between the lines representing the orbitals containing the bonded electrons, e.g. H-C-H bond angle in CH4 is 109°28′ and F-B-F bond angle in BF3 is 120°.
- Bond order. It is the number of bonds between two atoms in a molecule, e.g. in H-H (H2) the bond order is one, in O = O(O2), it is two.