MP Board Class 12th Chemistry Solutions Chapter 7 The p-Block Elements
The p-Block Elements NCERT Intext Exercises
Question 1.
Why are.pentahalides more covalent than trihalides ?
Answer:
Higher the positive oxidation state of central atom, it will have more polarising power which, in turn will increase the covalent character of the bond formed between the central atom and the other atom. Hence pentahalides are more covalent than trihalide.
Question 2.
Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements ?
Answer:
Among the hydrides of Group-15, BiH3 is the least stable because Bi has largest size in group and has least tendency to form covalent bond with small hydrogen atom. Therefore, it can readily lost H atom and has strongest tendency to act as reducing agent.
Question 3.
Why is N2 less reactive at room temperature?
Answer:
In N2 molecule, there is a strong pπ-pπ overlap that results in the formation of triple bond between N atoms (N ≡ N). As a consequence, the bond dissociation energy of N2 is very high and N2 is less reactive at room temperature.
Question 4.
Mention the conditions required to maximise the yield of ammonia.
Answer:
Ammonia is prepared by the Haber’s process.
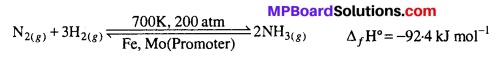
In accordance with Le-chatelier’s principle, to maximize the yield, a high pressure 200 atm is used. To increase the rate of the reaction, a temperature of around 700 K is used and iron oxide mixed with some K2O and Al2O3 is used as a catalyst. Sometimes, Mo is also used as a promoter to increase the efficiency of the Fe catalyst.
Question 5.
How does ammonia reacts with a solution of Cu2+?
Answer:
Ammonia reacts with a solution of Cu+2 ions of solution to form deep blue coloured complex, tetra’amine copper (II) ion.

Question 6.
What is the covalence of nitrogen in N2O5 ?
Answer:
Covalency depends upon the number of shared pairs of,electrons. Now in N2O5, each nitrogen atom has four shared pairs of electrons as shown :
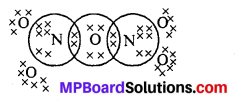
Therefore, the covalency of N in N2O5 is 4.
Question 7.
Bond angle in PH4+ is higher than that in PH3. Why ?
Answer:
PH4+ is a regular tetrahedral structure, thus bond angle is 109°28′.
PH3 molecule has a pyramidal structure. The bond angle is 93°. Hence, bond angle in PH4+ ion is higher.
Question 8.
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2 ?
Answer:
When white phosphorus is heated with cone. NaOH solution in an inert atmosphere of CO2, phosphine gas is prepared.
![]()
![]()
Question 9.
What happens when PCl5 is heated ?
Answer:
PCl5 has three equatorial and two axial bonds. Since axial bonds are weaker than equatorial bonds, therefore, when PCl5 is heated, the less stable axial bond breaks to form PCl3.
![]()
Question 10.
Write a balanced equation for the hydrolytic reaction of PCl5 in heavy water.
Answer:
PCl5 + D2O → POCl3 + 2DCl.
Question 11.
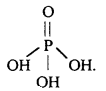
What is the basicity of H3PO4?
Answer:
H3PO4 contains three P-OH bonds and therefore, its basicity is three.
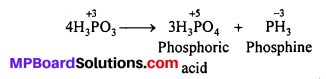
Question 12.
What happens when H3PO3 is heated ?
Answer:
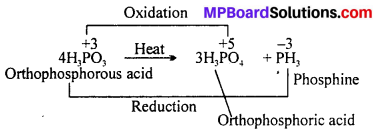
On heating, H3PO3 disproportionates to give orthophosphoric acid and phosphine.
Question 13.
List the important sources of sulphur.
Answer:
Sulphates and sulphides are important sources of sulphur.
Sulphates : Gypsum (CaSO4.2H2O), Epsom salt (MgSO4.7H2O), Baryte (BaSO4).
Sulphides : Galena (PbS), Zinc blende (ZnS), Copper pyrites (CuFeS2), Iron pyrites (FeS2) etc.
Organic materials such as eggs, proteins, garlic, onion, mustard, hair and wool also contain sulphur in small quantities.
Question 14.
Write the order of thermal stability of the hydrides of Group-16 elements.
Answer:
As the size of element increases down the group, the E-H bond dissociation energy decreases and hence E-H bond breaks more easily. Thus, the thermal stability of the hydrides of Group 16 elements decreases down the group, i.e., H2O > H2S > H2Se > H2Te > H2PO.
Question 15.
Why is H2O a liquid and H2S a gas ?
Answer:
Due to high electronegativity of oxygen and its small size, there are strong H-bonding in water. As a result, the molecules exist as associated and is liquid at room temperature. But there is no H-bonding in H2S because of low electronegativity of ‘S’.
Question 16.
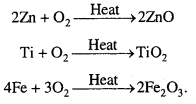
Which of the following does not react with oxygen directly ?
Zn, Ti, Pt, Fe
Answer:
Being noble metal, Pt does not react with oxygen directly. Zn, Ti and Fe are active metals so they react with oxygen.
Question 17.
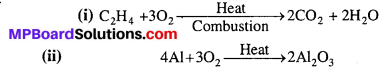
Complete the following reactions :
(i) C2H4 + O2 →
(ii) 4Al + 3O2 →
Answer:
Question 18.
Why does O3 acts as a powerful oxidizing agent ?
Answer:
O3 is an endothermic compound. On heating, it readily decompose to give dioxygen and nascent oxygen.
![]()
Since it liberates nascent oxygen easily. It acts as powerful oxidising agent.
Question 19.
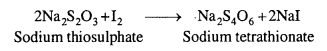
How is O3 estimated quantitatively ?
Answer:
Ozone (O3) reacts with an excess of potassium iodide (KI) solution buffered with a borate buffer (pH = 9-2) and liberate (I2).
2I– + H2O(l) + O3(g) → 2OH–(aq) + I2(s) + O2(g)
The liberated I2 is titrated against a standard solution of sodium thiosulphate. At the end point, I2 combines with the starch to give the deep blue starch-iodine complex.
This method is also used for detecting ozone in polluted air.
Question 20.
What happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt ?
Answer:
Sulphur dioxide (a reducing agent) reduces Fe (III) ions to Fe (II) ions as per the following reaction:
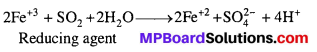
Question 21.
Comment on the nature of two S-0 bonds formed in SO2 molecule. Are the two S-O bonds in this molecule equal ?
Answer:
Both the S-0 bonds are covalent and have equal strength due to resonating structures.
![]()
Question 22.
How is the presence of SO2 detected ?
Answer:
The presence of SO2 detected by following ways :
(a) SO2 (reducing agent) decolourises acidified potassium permanganate solution.
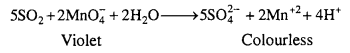
(b) SO2 turns orange coloured acidified K2Cr2O7 solution green due to reduction of Cr2O72- to Cr+3 ions.
![]()
Question 23.
Mention three areas in which H2SO4 plays an important role.
Answer:
Uses of H2SO4 :
(i) Manufacture of fertilizers (e.g. ammonium sulphate, calcium superphosphate)
(ii) Petroleum refining
(iii) Metallurgical applications (e.g. cleansing metals before enamelling electroplating, galvanising etc.)
Question 24.
Write the conditions to maximise the yield of H2SO4 by Contact process.
Answer:
![]()
According to Le-chatelier’s principle following are the requirements for maximum yield.
(i) Low temperature as the conversion of SO2 to SO3 is exothermic (720K).
(ii) High pressure (2 atm).
(iii) A catalyst (Pt or V2O5) to increase the rate of reaction.
Question 25.
Why is Ka2 << Ka1 for H2SO4 in water ?
Answer:
H2SO4 is a dibasic acid, it ionizes in two stages and hence has two dissociation constants.
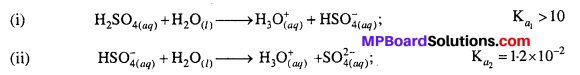
Ka2 is less than Ka, because the negatively charged HSO–4 ion has much less tendency to denote a proton to H2O as compared to neutral H2SO4 due to electrostatic reason.
Question 26.
Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidizing power of F2 and Cl2.
Answer:
Oxidising power is a combined effect of bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy. Comparing F2 and Cl2 with the given parameters :

From the data given above, it is clear that bond dissociation enthalpy and electron gain enthalpy are higher for chlorine but hydration enthalpy is much higher for fluorine. It compensates the effect of other two. As a result, the ∆H overall is more negative for F2 than for Cl2. Hence, F2 is stronger oxidizing agent than Cl2.
Question 27.
Give two examples to show the anomalous behaviour of fluorine.
Answer:
(i) Two anomalous behaviour of fluorine are : (i) Since fluorine is most elec-tronegative element, it shows only a negative oxidition state of-1. It does not show any positive oxidation state. On the other hand, the other halogens show positive oxidation states also such as +1, +3, +5, +6 and +7.
(ii) Maximum co valency of fluorine is one because it can not expand its valence shell beyond octet because there are no d-orbitals in the valence shell. On the other hand, other elements can exercise covalencies up to 7 because of availability of vacant d-orbitals.
Question 28.
Sea is the greatest source of some halogens. Comment.
Answer:
Sea water contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium, but mainly sodium chloride (2-5% by mass). Dried up sea-weeds contain sodium chloride and carnallite, KCl. MgCl2. 6H2O. Certain sea-weeds contain up to 0-5% of iodine as sodium iodide and chile saltpetre (NaNO3) contains up to 0-2% of sodium iodate. Thus, sea is the greatest source of halogen.
Question 29.
Give the reason for bleaching action of Cl2.
Answer:
Bleaching action of chlorine is due to its oxidation. In the presence of moisture, chlorine gives nascent oxygen.
Cl2 + H2O → 2HCl + [0]
Because of nascent oxygen, it bleaches colouring substance as
Colouring substance +[0] → Colourless substance.
It bleaches vegetables or organic matter. The bleaching action of chlorine is permanent.
Question 30.
Name two poisonous gases which can be prepared from chlorine gas.
Answer:
(i) Phosgene (COCl2)
(ii) Tear gas (CCl3.NO2)

Question 31.
Why is I-Cl more reactive than I2 ?
Answer:
I-Cl bond is weaker than I-I bond, so ICl is more reactive than I2.
Question 32.
Why is Helium used in diving apparatus ?
Answer:
Helium is used as a diluent for oxygen is modern diving apparatus because of its very low solubility in blood.
Question 33.
Balance the following equation :
XeF6 + H2O → XeO2F2 + HF
Answer:
XeF6 + 2H2O → XeO2F2 + 4HF.
Question 34.
Why has it been difficult to study the chemistry of Radon ?
Answer:
Radon is a radioactive with very short half life of 3-82 day’s that’s why the study of Chemistry of Radon is difficult.
![]()
The p-Block Elements NCERT TextBook Exercises
Question 1.
Discuss the general characteristics of Group-15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Answer:
The group-15 of the periodic table contains five elements, namely nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi). These elements are called ‘Pnicogens’ and their compounds as ‘Pniconides’. The name is derived from the Greek word ‘Pnicogens’ meaning suffocation.
Its general characteristics are given below :
1. Electronic configuration : these elements have five valency electrons therefore, their general valence shell electronic configuration will be ns2 np3, where, n = 2 to 6. The penultimate shell has 2 electrons in case of nitrogen, 8 electrons in phosphorus and 18 electrons in other elements.
In accordance with the Hund’s rule of maximum multiplicity the three np electrons are distributed as np1x, np1y, np1z. Thus, the p-orbitals are half filled and hence are stable and do not show much reactivity.
2. Oxidation state: The valency shell electronic configuration ns2 np3 of these elements suggests that these elements can show an oxidation states of 3, +3 and +5.
Nitrogen and phosphorus the first two members show negative oxidation state of -3 in their compound. Since, their electronegativities are high and atomic size are small. They form anions such as nitride ion (Mg3N2) and phosphide ion (Mg3P2) with highly positive elements. But, this tendency to show negative oxidation state decreases down the group due to the decrease in electronegativity and increase in size of the elements.
All these elements show positive oxidation state when they combine with more electronegative elements. They show +3 and +5 oxidation states in their compounds. However, the stability of +3 oxidation state increases and that of +5 oxidation state decreases down the group on account of inert pair effect (in ability of ns electrons to participate in bonding). This is noticed maximum in Bi due to very large nuclear charge. Thus, a molecule of BiCl3 can exist and not of BiCl5.
3. Atomic size: The atomic radii (covalent) and ionic radii (in same oxidation state) of 15th group members are smaller as compared to 14th group elements.
Reason : As we move left to right in a period nuclear charge increases and the new electrons are added in the same shell. Due to that effective nuclear charge increases (Z-effective). This results in decrease in atomic and ionic radii.
On going down the group, the covalent and ionic radii (in a same oxidation state) increases with the increase in atomic number. This increase is very large from N to P. However, from As to Bi only a small increase is observed.
Reason: On moving down the group increase in size can be explained on the basis of successive addition of new shell due to that Z-effective decreases. But, after ‘P’ increase in size is comparatively very small, because of insufficient shielding effect of 3d-electrons (Z- effect increases). Similarly, increase in size from Sb to Bi is small due to insufficient screening by 4/-electrons.
4. Ionization enthalpy : (a) The elements of group 15th element (nitrogen family) have sufficiently high ionization enthalpy which are more than the corresponding elements of group 14th element (carbon family).
(b) Down the group, the value of ionization enthalpies decreases regularly.
5. Electronegativity : As compared to group 14th element, group 15th elements are more electronegative. The electronegativity value decreases on moving down the group from N to Bi. Nitrogen is the most electronegative element of the family.
Question 2.
Why does the reactivity of nitrogen differ from phosphorus ?
Answer:
Molecular nitrogen exist as a diatomic molecule having a triple bond between the two nitrogen atoms, N = N (due to it stability to form pπ-pπ multiple bonding). The bond dissociation energy is very high (941-4 kJ mol-1). Thus, under ordinary conditions, nitrogen behaves as an inert gas. On the other hand, white and yellow phosphorus exists as a triatomic molecule (P4) having single bonds. The dissociation energy of P-P bond is low (213 kJ mol-1). Thus, phosphorus is much more reactive than nitrogen.
Question 3.
Discuss the trends in chemical reactivity of Group 15th elements.
Answer:
Chemical reactivity: The elements of group 15 show different reactivity. Nitro¬gen, inspite of its greater electronegativity value of 3, is chemically inert.
The chemical inertness of nitrogen is due to the presence of a triple bond between two nitrogen atoms (N ≡ N) which is very strong and requires high dissociation energy (941.4 kJ mol-1).
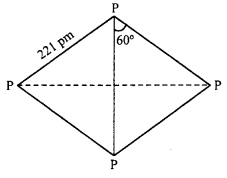
Phosphorus exists in P4 tetrahedral molecules, each p-p-p angle is 60° instead of 109°28 ‘as expected from sp3 hybridization. Thus P4 molecule has a highly strained cage like structure and this makes white phosphorus highly reactive, (on the other hand red phosphorus has opened up network structure and not discrete P4 molecule hence less reactive than white phosphorus). As, Sb and Bi are also not very reactive.
Question 4.
Why does NH3 form hydrogen bond but PH3 does not ?
Answer:
The N – H bond in ammonia is quite polar as nitrogen is highly electronegative in nature. As a result, NH3 molecules are linked by intermolecular hydrogen bonding.
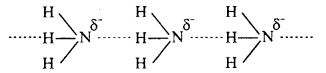
On the other hand, P – H bond is non-polar as both P and H have same electronegativ¬ity. Hence in phosphine no hydrogen bonding is present.
Question 5.
How is nitrogen prepared in the laboratory ? Write the chemical equations of the reactions involved.
Answer:
(i) In the laboratory, dinitrogen is prepared by heating an aqueous solution of ammonium chloride with sodium nitrite.
NH4Cl(ag) + NaNO2(aq) → N2(g) + 2H2O(l) + NaCl(aq)
(ii) Preparation by thermal decomposition of Ammonium dichromate.
![]()
(iii) Net N2 is obtained from sodium or Barium azide.
![]()
Question 6.
How is ammonia manufactured industrially ?
Answer:
Theory : One volume of nitrogen and three volume of hydrogen reacts together to form ammonia. It is an exothermic process. Formation of ammonia is accompanied by decrease in volume because four volume of reactants react to form two volumes of product. Thus, according to Le-chatelier’s principle high concentration of N2 and H2 low temperature and high pressure is favourable for the formation of ammonia.
Chemical reaction:
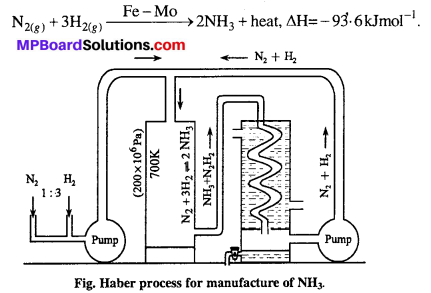
Method: Pure nitrogen and pure and dry hydrogen are taken in ratio 1:3. The above mixture is passed in chamber containing ferric oxide (Fe2O3) as catalyst and molybdenum as promoter at 400-500C under the high pressure of 200 atmospheric pressure. The gas obtained after reaction contains 15-40% is cooled by condensers and liquid ammonia is obtained.
![]()
Precautions : (i) N2 and H2 should be pure and dry because impurities damage catalytic activities and
(ii) Temperature and pressure should be controlled.
Question 7.
Illustrate how copper metal can give different products on reaction with HNO3.
Answer:
On heating with dil.HNO3, Copper gives copper nitrate and nitric oxide.
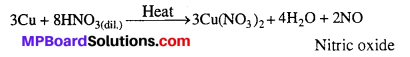
With cone. HNO3, instead of NO, NO2 is evolved.
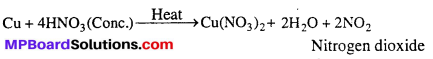
Question 8.
Give the resonating structures of NO2 and N2O5.
Answer:
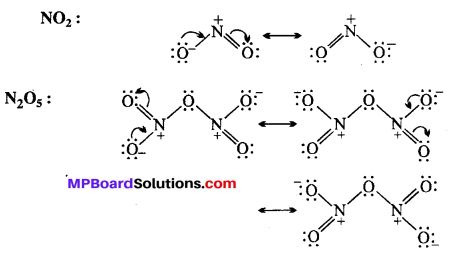
![]()
Question 9.
The HNH angle value is higher than HPH, HAsH and HSbH angles. Why?
[Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s-p bonding between hydrogen and other elements of the group].
Answer:
According to VSEPR theory, the lone pair-bond pair repulsion is larger than bond pair-bond pair repulsion. As a result, the tetrahedral shape is distorted and the bond angle becomes less than tetrahedral angle (109°28′). Electronegativity of N is maximum in group 15, so bond pairs of electrons lie much closer to N in NH3. The force of repulsion between the adjacent bond pairs are also high so the HNH bond angle value is higher in NH3 than HPH, HAsH and HSbH angles.
Question 10.
Why does R3P = O exist but R3N = O does not (R = alkyl group)?
Answer:
Nitrogen does not have d-orbitals in its valence shell. Therefore, it can not extend its covalency to five by forming dπ-dπ bonding. As a result, the molecule of R3N = O does not exist. However, phosphorus has vacant rf-orbitals in the valence shell and can form dx-dxbonding. Thus, a molecule like R3P = O can exist.
Question 11.
Explain why NH3 is basic while BiH3 is only feebly basic.
Answer:
Atomic size of N(70pm) is much smaller than that of Bi (148 pm). Therefore, electron density on the N-atom is much higher than that on Bi-atom. Consequently, the tendency of N in NH3 to donate the pair of electrons is much higher than that of Bi in BiH3. Thus, NH3 is basic while BiH3 is only feebly basic.
Question 12.
Nitrogen exists as diatomic molecule and phosphorus as P4 Why?
Answer:
Nitrogen because of its small size and high electronegativity is capable of forming pπ-pπ multiple bonding. Therefore, it exists as diatomic molecule with one σ and two K bonds (N ≡ N). Phosphorus, on the other hand, has a longer size and lower electronegativity and thus is not capable to form pπ-pπ multiple bonds. It prefers to form single bonds hence, it exists as tetrahedral P4 molecules.
Question 13.
Write main differences between the properties of white phosphorus and red phosphorus.
Answer:
Comparison of properties of White and Red phosphorus :
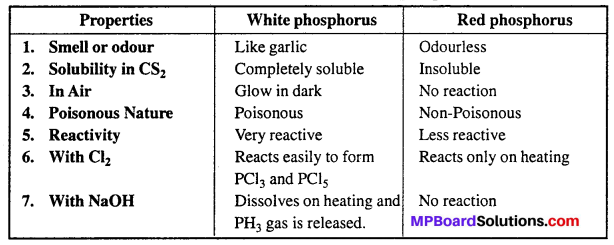
Question 14.
Why does nitrogen show catenation properties less than phosphorus?
Answer:
The catenation properties depends upon the strength of the element-element bond. The N-N bond strength is much weaker (due to repulsion of lone pairs on nitrogen because of its small size) than the P-P bond strength, therefore, nitrogen shows catenation less than phosphorus.
Question 15.
Give the disproportionation reaction of H3PO3.
Answer:
Question 16.
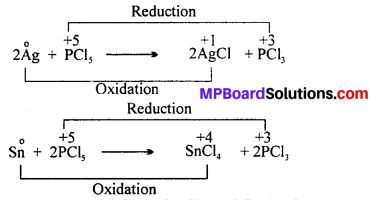
Can PCl5 acts as an oxidising as well as a reducing agent ? Justify.
Answer:
Phosphorus has maximum oxidation state of +5 in PCl5. It can not increase its oxidation state further and thus it can not act as reducing agent. However, PCl5 can act as an oxidising agent as it can itself reduce from +5 to +3 oxidation state. For example, PCl5 oxidises Ag and Sn in the following reactions :
Question 17.
Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Answer:
The elements of group 16 are collectively called chalcogens.
(i) Elements of group 16 have six valence electrons each. The general electronic configuration of these elements is ns2 np4, where n varies from 2 to 6.
(ii) Oxidation state : As these elements have six valence electrons (ns2np4), they should display an oxidation state of -2. However, only oxygen predominatntly shows the oxidation state of-2 owing to its high electronegativity. It also exhibits the oxidation state of -1(H2O2), zero (O2) and +2 (OF2).
However, the stability of the -2 oxidation state decreases on moving down a group due to a decrease in the electronegativity of the elements. The heavier elements of the group show an oxidation state of +2, +4 and +6 due to the availability of d-orbitals.
(iii) Formation of hydrides : These elements from hydrides of formula H2E, where E = O, S, Se, Te, Po. Oxygen and sulphur also form hydrides of type H2E2. These hydrides are quite volatile in nature.
Question 18.
Why is dioxygen a gas but sulphur a solid?
Answer:
The oxygen atom has tendency to form pπ-pπ multiple bonding due to its small size and high electronegativity. As a result, oxygen exist as diatomic molecule. These mol¬ecules are held together by weak van der waal’s forces of attraction which can be easily overcome by collisions of the molecules at room temperature. Therefore, O2 is the gas at room temperature.
Sulphur, on the other hand, because of its bigger size and lower electronegativity, does not form pπ-pπ multiple bonds. Instea it prefers to form S-S single bond and form polyatomic complex molecules having eight atoms per molecule (S8) and have puckered ring structure. Therefore, S atoms are strongly held together and it exists as a solid.
![]()
Question 19.
Knowing the electron gain enthalpy values for O → O– and O → O2- as -141 and 702 kj mol-1 respectively, how can you account for the formation of a large number of oxides having O2- species and not O– ?
(Hint: Consider lattice energy factor in the formation of compounds).
Answer:
Although the formation of O2- anions requires more energy in comparison to the formation of O– anion (actually energy is released). Yet in large number of oxides, oxygen is divalent in nature. This is due to the fact that lattice energies of the oxides having O2- anions are very high on account of greater magnitude of electrostatic force of attraction.
Question 20.
Which aerosols deplete ozone ?
Answer:
Freon (CCl2F2) (Chlorofluoro carbon).
Question 21.
Describe the manufacture of H2SO4 by contact process.
Answer:
Manufacture of sulphuric acid : Sulphuric acid is prepared on large scale by contact process. The basic raw material used is either sulphur or iron pyrites.
Principle of contact process : When pure and dry SO2 mixed with air is passed over V2O5 catalyst it gets oxidised to SO3 which is absorbed by H2SO4 to form oleum i.e., H2S2O7.
2SO2 + O2 → 2SO3 + 45.2 cal
SO3 + H2SO4 → H2S2O7
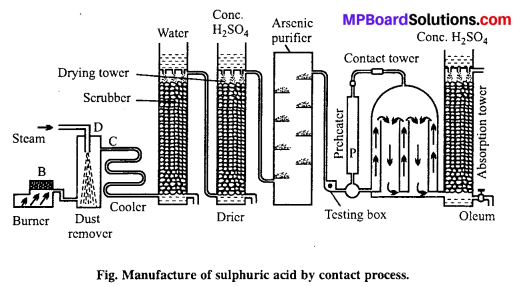
Details of the process are given below :
(a) Sulphur or pyrite burners : SO2 is produced by burning sulphur or roasting pyrites in pyrite burners.
(b) Purification unit: This unit is an assembly of various parts like.
(i) Dust chamber: SO2 gas produced is passed through dust chamber through which steam is sprayed from top. Dust particles absorb moisture, become heavy and settle down.
(ii) Cooling pipes : Now, the gaseous mixture is passed through cooling pipes to lower the temperature to 100°C.
(iii) Washing tower or scrubbers : It is filled with quartz and showers of cold water continue from top. Dust particles as well as soluble impurities settle down and are removed.
(iv) Drying tower : It is a high tower full of quartz pieces. Cone. H2SO4 is sprayed from the top. Gases enter the tower from bottom and get dried in contact with cone. H2SO4.
(v) Arsenic purifier : It is filled with shelves containing freshly prepared ferric hydroxide which absorb the impurities of arsenic purified and dried.
(c) Testing box : Before sending the gaseous mixture to contact chamber it is tested here. A strong beam of light is thrown into the testing box, if the gaseous mixture contains dust or any other particles they become visible, hence gaseous mixture is repurified. The completely pure gas is then sent to the contact chamber.
(d) Preheater : Pure gas free from dust particle is heated in preheater to 450°C and then passed into contact tower, where SO2 gets oxidized to SO3. This reaction is exothermic due to which temperature of contact tower raises to 450°C. After this the pure gases are pumped directly into the contact tower.
(e) Contact tower : It consists of a big iron container containing several pipes filled with vanadium pentoxide or platinized asbestos or any other suitable catalyst. Temperature is maintained at 450°C. Pure and dry SO2 reacts with air in these pipes to form sulphur trioxide.
![]()
On account of exothermic nature of reaction, sufficient heat is available.
(f) Absorption tower: The sulphur trioxide is then passed into a tower in which cone, sulphuric acid flows down in the form of spray. Sulphuric acid absorbs SO3 and becomes more concentrated. This acid, due to excess of SO3 produces a fog in the tower. The acid obtained is called fuming sulphuric acid or oleum.
![]()
Oleum is mixed with calculated quantity of water to form acid of a particular strength.
H2S2O7 + H2O → 2H2SO4
It may be noted that sulphur trioxide is not directly absorbed in water to form sulphuric acid because it forms a dense fog of sulphuric acid which does not condense easily.
Note : For detail refer to your NCERT Text-Book.
Question 22.
How is SO2 an air pollutant?
Answer:
SO2 dissolves in rain water and produces acid rain. The acid rain contains sulphuric acid
![]()
In addition to H2SO4, acid rain also contains HNO3.
Question 23.
Why are halogens strong oxidising agents?
Answer:
Halogens are strong oxidising agents due to their low bond dissociation enthalpy, high electronegativity and large negative electron gain enthalpy. Halogens have a strong tendency to accept electrons and thus get reduced.
X2 + 2e– → 2X–
As a result, halogen acts as strong oxidising agents. Their oxidising power however decreases from F2 to I2 as is evident from their electrode potentials:

Question 24.
Explain why fluorine forms only one oxyacid, HOF ?
Answer:
Due to absence of d-orbital, fluorine does not shqw +3, +5 and + 7 oxidation state, while other halogen do show. Thus, it does not form oxoacids like HOFO, HOFO2, HOFO3. It shows only +1 oxidation state and form HOF only.
Question 25.
Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Answer:
Oxygen has smaller size than chlorine, smaller size of oxygen favours hydrogen bonding.
Question 26.
Write two uses of ClO2.
Answer:
Two uses of ClO2 :
(i) It is a powerful oxidising and chlorinating agent.
(ii) It is an excellent bleaching agent for wood pulp, flour for making white bread.
Question 27.
Why are halogens coloured ?
Answer:
Halogen absorb part of light in the visible region which causes excitation of outer electrons to higher energy levels. By absorbing different quanta of radiations, they display different colours. Fluorine atom is the smallest and the force of attraction between the nucleus and the outer electrons is very large. As a result, it requires large excitation energy and absorbs violet light and therefore appear yellow (complementary colour). As different colours are absorbed by different halogens, they display different complementary colour also.
Question 28.
Write the reactions of F2 and CI2 with water.
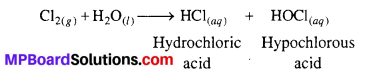
Answer:
F2 being strong oxidising agent oxidises H2O to O2 or O3.

Cl2, on the other hand, reacts with H2O to form hydrochloric acid and hypochlorous acid.
Question 29.
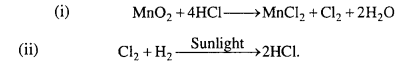
How can you prepare Cl2 from HCl and HCl from Cl2? Write reactions only.
Answer:
Question 30.
What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Answer:
Neil Bartlett observed that PtF6 reacts with O2 to form an ionic solid O+2 PtF6
O2(g) + PtF6(g) → O+2[PtF6]–
In this reaction, O2 gets oxidised to O+2 by PtF6.
Since the first ionisation energy of xenon is fairly close to that of oxygen. Bartlett thought that PtF6 should also oxidise Xe to Xe+. This prompted Bartlett to carry out the reaction between Xe and PtF6 and formed the first noble gas compound.
![]()
Question 31.
What are the oxidation states of phosphorus in the following :
(i) H3PO3
(ii) PCl3
(iii) Ca3P2
(iv) Na3PO4
(v) POF3 ?
Answer:
(i) H3PO3 = 3 × (+1) + 3 × (-2) + X = 0 or X = +3
(ii) PCl3 = X + 3(-1) = 0 or X = +3
(iii) Ca3P2 = 3 × (+2) + 2 X = 0 or X = -3
(iv) Na3PO4 = 3 × (+1) + X + 4 × (-2) = 0 or X = +5
(v) POF3 = X (-2) + 3 × (-1) = 0 or X = +5.
![]()
Question 32.
Write balanced equations for the following :
(i) NaCI is heated with sulphuric acid in the presence of MnO2.
(ii) Chlorine gas is passed into a solution of Nal in water.
Answer:
(i) Cl2 is produced
4NaCl + MnO2 + 4H2SO4 → MnCl2 + 4NaHSO4 + 2H2O + Cl2
(ii) Cl2 being an oxidising agent oxidises Nal to I2.
Cl2(g) + 2NaI(aq) → 2NaCl(aq) + I2(s)
Question 33.
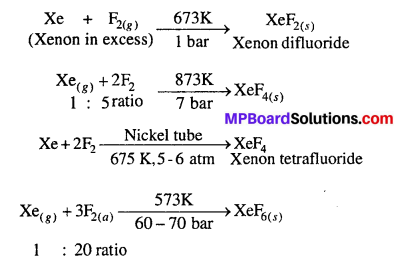
How are xenon fluorides XeF2, XeF4 and XeF6 obtained ?
Answer:
Xenon Fluorides : Three fluorides of xenon are important. These are xenon difluoride (XeF2), xenon tetrafluoride (XeF4) and xenon hexafluoride (XeF6).The fluorides of xenon can be prepared by the direct combination between xenon and fluorine under different conditions.
Question 34.
With what neutral molecule is CIO– isoelectronic? Is that molecule a Lewis base ?
Answer:
OF2 and ClF are isoelectronic to CIO–, out of which ClF is a Lewis base.
Question 35.
How are XeO3 and XeOF4 prepared ?
Answer:
Hydrolysis of XeF4 and XeF6 with water forms XeO3.
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
XeF6 + 3H2O → XeO3 + 6HF
Partial hydrolysis of XeF6 gives XeOF4.
XeF6 + H2O → XEOF4 + 2HF
Question 36.
Arrange the following in the order of property indicated for each set:
(i) F2, Cl2, Br2,I2 – increasing bond dissociation enthalpy.
(ii) HF, HCl, HBr, HI – increasing acid strength.
(iii) NH3, PH3, ASH3, SbH3, BiH3 – increasing base strength.
Answer:
(i) I2 < F2 < Br2 < Cl2
![]()
Here F2 has very less bond dissociation energy inspite of having small size. It is due to repulsion be¬tween the lone pair of electrons on small size F-atoms.
(ii) HF < HCl < HBr < HI
It depends upon their bond dissociation enthalpy, which decreases from H-F to H-I as the size of atom increases from F to I.
(iii) BiH3 < SbH3 < AsH3 < PH3 < NH3.
They are lewis bases due to presence of lone pair of electrons on central atom of hydrides. The availability of lone pair of electrons is maximum in nitrogen owing to its small size therefore, NH3 is maximum basic and as the size of central atom increases availability decreases.
Question 37.
Which one of the following does not exist ?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) XeF6
Answer:
NeF2 does not exist as Ne does not have J-orbitals in valence shell and possesses high ionisation enthalpies. Thus, there is no scope of promotion of electrons i.e. formation of half filled orbitals. Hence, the formation of NeF2 is not possible.
Question 38.
Give the formula and describe the structure of a noble gas species which is isostructural with:
(i) ICl4–
(ii) IBr2–
(iii) BrO3–.
Answer:
(i) ICl4– : sp3d2 hybridisation, square planar shape.
XeF4 is an isostructural species.
(ii) IBr2– : sp3d hybridisation, Linear shape.
XeF2 is an isostructural species.
(iii) BrO3–: sp3 hybridisation, tetrahedral geometry (Pyramidal shape)
XeO3 is an isostructural species.
Question 39.
Why do noble gases have comparatively large atomic sizes?
Answer:
In case of noble gases, the atomic radii corresponds to van der Waal’s radii, which are always large.
Question 40.
List the uses of neon and argon gases.
Answer:
Uses of Neon : (i) Neon lights are used for commercial advertisements. It consists of a long tube fitted with electrodes at both ends. On filling the tube with neon gas and passing electric discharge of about 1000 volt potential, a bright red light is produced. Different colours can be obtained by mixing neon with other gases. For producing blue or green light neon is mixed with mercury vapours.
Uses of Argon :
(i) Like helium, it is also used to create inert atmosphere in welding of aluminium ahd stainless steel.
(ii) It is filled in electric bulbs along with 25% nitrogen.
(iii) It is also used in radio valves.
(iv) Argon alone or its mixture with neon is used in tubes for producing lights of different colours.
![]()
The p-Block Elements Other Important Questions and Answers
The p-Block Elements Objective Type Questions
Question 1.
(A) Choose the correct answer :
Question 1.
In which compound oxygen exhibits +2 oxidation state :
(a) H2O
(b) Na2O
(c) OF2
(d) MgO.
Answer:
(c) OF2
Question 2.
Reddish brown gas is formed, when nitric oxide oxidizes by air. This gas is :
(a) Na2O2
(b) Na2O4
(c) NO2
(d) N2O3.
Answer:
(c) NO2
Question 3.
H3PO3 is :
(a) Dibasic acid
(b) Monobasic acid
(c) Tribasic acid
(d) Tetrabasic acid
Answer:
(c) Tribasic acid
Question 4.
Which one of the following is a typical metal:
(a) P
(b) As
(c) Sb
(d) Bi.
Answer:
(d) Bi.
Question 5.
Oxide which shows paramagnetic character :
(a) N2O4
(b) NO4
(C) P4O6
(d) N2O5.
Answer:
(b) NO4
Question 6.
Ammonia can be dried by :
(a) H2SO4
(b) P2O5
(c) Anhydrous CaCl2
(d) CaO.
Answer:
(d) CaO.
Question 7.
Nitric acid change iodine into :
(a) Iodic acid
(b) Hydroiodic acid
(c) Iodine pentaoxide
(d) Iodine nitrate.
Answer:
(a) Iodic acid
Question 8.
NH3 is a Lewis base which forms complex salt with cations. Following cation does not form complex salt with NH3 :
(a) Ag+
(b) Cu2+
(c) Cd2+
(d) Pb2+
Answer:
(d) Pb2+
Question 9.
Ammonia is soluble in:
(a) Hg2Cl2
(b) PbCl2
(c) Agl
(d) Cu(OH)2.
Answer:
(d) Cu(OH)2.
Question 10.
How much water molecules are required to change one molecule of P2O5 into orthophosphoric acid:
(a) 2
(b) 3
(c) 4
(d) 5.
Answer:
(b) 3
Question 11.
Bleaching action of SO2 is due to :
(a) Reduction
(b) Oxidation
(c) Hydrolysis
(d) Acidic nature.
Answer:
(a) Reduction
Question 12.
What happens when SO2 is passed through acidic K2Cr2O7 :
(a) Solution turns blue
(b) Solution turns colourless
(c) SO2 reduced
(d) Green chromic sulphate is formed.
Answer:
(d) Green chromic sulphate is formed.
Question 13.
P2O3 forms which of the following acid :
(a) H4P2O7
(b) H3PO4
(C) H3PO3
(d) HPO3
Answer:
(C) H3PO3
Question 14.
Most acidic halide is :
(a) PCl5
(b) SbCl3
(c) BrCl3
(d) CCl4
Answer:
(a) PCl5
Question 15.
The catalyst used in manufacturing of H2SO4 is :
(a) Al2O3
(b) CrO3
(c) V2O5
(d) MnO2
Answer:
(c) V2O5
Question 16.
The hydrolysis of phosphorus trihalides give :
(a) One monobasic acid and one dibasic acid
(b) One monobasic acid and one tribasic acid
(c) One monobasic acid and a salt
(d) Two dibasic acids.
Answer:
(a) One monobasic acid and one dibasic acid
Question 17.
In the following reaction :
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
(a) P oxidises
(b) P oxidises and reduces
(c) P reduces
(d) Na oxidises.
Answer:
(b) P oxidises and reduces
Question 18.
Laughing gas is :
(a) NO
(b) N2O
(C) N2O3
(d) N2O5.
Answer:
(b) N2O
Question 19.
White phosphorus does not contain :
(a) 6 P-P single bond
(b) 4 P-P single bond
(c) 4 lone pair of electron
(d) Bond angle of P-P-P is 60°.
Answer:
(b) 4 P-P single bond
Question 20.
On heating NH4Cl and NaNO2 solution gives :
(a) N2O
(b) N2
(C) NO2
(d) NH3.
Answer:
(b) N2
Question 21.
Formula of metaphosphoric acid is :
(a) H3PO4
(b) HPO3
(c) H3PO3
(d) H2PO3.
Answer:
(b) HPO3
Question 22.
Gas which cannot be collected on water :
(a) Na
(b) O2
(c) SO3
(d) PH3.
Answer:
(c) SO3
(B) Choose the correct answer :
Question 1.
Chlorine shows bleaching property in the presence of:
(a) Dry air
(b) Moisture
(c) Sunlight
(d) Pure oxygen.
Answer:
(b) Moisture
Question 2.
Which of the following element forms least number of compounds :
(a) He
(b) Ar
(c) Kr
(d) Xe.
Answer:
(a) He
Question 3.
Which is used for bright advertisement display :
(a) Xe
(b) Ar
(c) Ne
(d) He.
Answer:
(c) Ne
Question 4.
Monazite is a source of:
(a) Ne
(b) Ar
(c) Kr
(d) He.
Answer:
(d) He.
Question 5.
Which of the following is not obtained by the direct reactions of the constituents:
(a) XeF2
(b) XeF4
(c) XeO3
(d) XeF6.
Answer:
(a) XeF2
Question 6.
Which of the following halides are least stable and whose existence is suspicious:
(a) CI4
(b) GeI4
(c) SnI4
(d) PbI4
Answer:
(d) PbI4
Question 7.
Which of the following is the strongest acid:
(a) HBr
(b) HCl
(c) HF
(d) HI.
Answer:
(d) HI.
Question 8.
Most electronegative element is:
(a) F
(b) Cl
(c) Br
(d) I.
Answer:
(a) F
Question 9.
Which halogen is solid at room temperature:
(a) Cl2
(b) I2
(c) Br2
(d) F2
Answer:
(d) F2
Question 10.
Which has maximum electron affinity:
(a) F
(b) Cl
(c) Br
(d) I.
Answer:
(b) Cl
Question 11.
Electronic configuration of valence shell of halogen ¡s:
(a) s2 p5
(b) s2p3
(c) s2p6
(d) s2p4.
Answer:
(a) s2 p5
Question 12.
Which element is most alkaline:
(a) F
(b) Cl
(c) Br
(d) I.
Answer:
(d) I.
Question 13.
The strongest reducing agent is:
(a) F–
(b) Br–
(c) I–
(d) I.
Answer:
(c) I–
Question 14.
Which of the following is weakest acid:
(a) HF
(b) HCI
(c) HBr
(d) HI.
Answer:
(a) HF
Question 15.
Oxidizing property ¡s highest of:
(a) I2
(b) Br2
(c) F2
(d) Cl2.
Answer:
(c) F2
Question 16.
Which Noble gas does not have octet complete:
(a) Helium
(b) Neon
(c) Argon
(d) Krypton.
Answer:
(a) Helium
Question 17.
Which Halogen sublimates:
(a) Chlorine
(b) Bromine
(c) Iodine
(d) Fluorine.
Answer:
(c) Iodine
Question 18.
Which of the noble gas is most soluble in water:
(a) He
(b) Ar
(c) Ne
(d) Xe.
Answer:
(d) Xe.
Question 19.
I2 easily dissolve in KI solution to form :
(a) I
(b) KI2
(C) KI
(d) KI3.
Answer:
(d) KI3.
Question 20.
Gas mixed in oxygen which is used in respiration for asthma patients :
(a) N2
(b) Cl2
(c) He
(d) Ne.
Answer:
(c) He
Question 21.
Deacon’s process is used for manufacture of:
(a) Bleaching powder
(b) Chlorine
(c) Nitric acid
(d) Sulphuric acid.
Answer:
(b) Chlorine
Question 22.
Sea grass is the source of industrial manufacture of:
(a) Chlorine
(b) Bromine
(c) Iodine
(d) Fluorine.
Answer:
(c) Iodine
Question 23.
Which gas is more useful to be filled in electric bulb :
(a) He
(b) Ne
(c) Ar
(d) Kr.
Answer:
(c) Ar
![]()
Question 2.
(A) Fill in the blanks :
1. N2O is a …………………. oxide.
2. Formula of Caro’s acid is ………………….
3. Concentrated nitric acid has brown colour due to the dissolution of …………………. gas.
4. Out of nitrogen oxides …………………. and …………………. are paramagnetic.
5. Pyrophosphoric acid is …………………. basic acid.
6. H2S gas cannot be dried by cone. H2SO4 because H2S …………………. it.
7. Fuming sulphuric acid dissolves in SO3 to form ………………….
8. Oxidation state of S in H2S2O8 (Marshall gas) is ………………….
9. NH3 gives white fumes of …………………. when it combines with HCl.
10. Elements of 16th group are known as ………………….
11. …………………. is used as a refrigerant.
Answers:
1. Neutral
2. H2SO5
3. NO2
4. NO, NO2
5. Tetra
6. reduces
7. Oleum
8. +6,9. NH4Cl
10. Chalcogen
11. Liquid NH3.
(B) Fill in the blanks :
1. ………………….. has the highest electron affinity.
2. In the presence of moisture, chlorine acts as a ………………….. agent.
3. Bleaching powder is also called as …………………..
4. At ordinary temperature bromine is a …………………..
5. Inter halogen compound AX5 has ………………….. structure.
6. Chlorine was discovered by …………………..
7. Neil Bertlett forms first noble compound which is …………………..
8. Element with highest electron affinity is …………………..
9. In oxy acid halogen present in ………………….. hybride state.
10. Gas which is light due to which it is filled in aircraft tyres is …………………..
11. ………………….. inert gas is mostly used in advertisements.
12. Elements of Group 17th are commonly known as …………………..
13. ………………….. is a radioactive inert gas.
Answer:
1. Chlorine
2. Bleaching
3. Calcium chlorohypochloride
4. Liquid
5. Square pyramidal
6. Scheele
7. Xe [PtF6]
8. Chlorine
9. sp3
10. Helium
11. Ne (Neon)
12. Halogen
13. Radon.
Question 3.
Match the following :
I.

Answers:
- (d)
- (c)
- (a)
- (b)
- (f)
- (g)
- (e)
- (i)
- (j)
- (h)
II.

Answers:
- (e)
- (c)
- (b)
- (a)
- (d).
III.

Answers:
- (e)
- (d)
- (b)
- (f)
- (c)
- (a).
![]()
Question 4.
(A) Answer in one word / sentence :
1. Who protects earth from ultraviolet rays ?
2. Give the chemical name of oil of vitriol or king of chemical.
3. Which gas is used for refrigeration ?
4. At which temperature water has maximum density ?
5. Give the name of compound formed by the dissolution of SO3 in sulphuric acid.
6. Write name of an antichlor.
7. Which gas is used for bleaching of oil and elephant teeth ?
8. Ammonium salts react with Nessler’s reagent to give which coloured precipitate ?
9. On moving down from N to Bi, +3 oxidation state of Bi becomes more stable than +5. Why ?
10. State the percentage of N2 by volume in nature.
Answers:
1. Ozone layer
2. H2SO4
3. NH3
4. 4°C
5. Oleum
6. SO2
7. Ozone
8. Brown
9. Due to Inert pair effect
10. 80%.
(B) Answer in one word/sentence :
1. Give the name of radioactive halogen.
2. Which noble gas is used in bulb with nitrogen ?
3. Write the name of noble gas which is used in treatment of cancer.
4. Write the formula of Carnalite.
5. Which noble gas is maximum available in the atmosphere ?
6. What is the shape of XeF6 ?
7. Write one use of fluorine.
8. Which type of hybridization is present in XeO3?
9. Write the oxidation state of F.
10. Write only equation for the preparation of chlorine in the lab.
11. What is the shape of AX3 type of Interhalogen compound ?
12. Write the correct order of strength of halogen acid.
13. Which noble gases do not form compounds ?
14. With which noble gas does F form compounds ?
15. Sea weeds are the source of which halogen ?
Answer:
1. Astatine
2. Ar
3. Rn
4. KCl.MgCl2.6H2O
5. Argon
6. Distorted octahedron
7. In preparing fluorocarbon, which is used in refrigeration
8. sp3
9. -1
10. MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
11. T- shape
12. HF < HCl < HBr < HI
13. He, Ne and Ar
14. Xe
15. Iodine.
![]()
The p-Block Elements Very Short Answer Type Questions
Question 1.
Why is dinitrogen (N2) less reactive at room temperature ?
Answer:
Due to high bond enthalpy of N ≡ N bond, dinitrogen is very less reactive at room temperature.
Question 2.
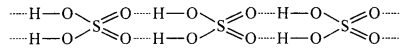
Why is concentrated sulphuric acid a high boiling point oily liquid ?
Answer:
Due to hydrogen bond between H2SO4 molecules, it is a high boiling point oily liquid.
Question 3.
Name two poisonous gases which are prepared from chlorine.
Answer:
(i) Phosgene (COCl2)
(ii) Tear gas (CCl3NO2).
Question 4.
Write the order of acidic strength of HCIO, HBrO and HIO.
Answer:
Strength of hypohalous acids decreases from HCIO to HIO.
HCIO > HBrO > HIO.
Question 5.
What are clathrate compounds ?
Answer:
In the cavity or space of crystal lattice of a compound small sized elements like noble gases get trapped within. Such compounds are called clathrate compounds.
Example : Kr3 (β-quinol).
Question 6.
Electronegativity of F atom is more than I atom still acidic strength of HF is less than HI. Explain.
Answer:
Due to small size of F atom, bond dissociation energy of HF bond is much higher than HI bond, in which size of I atom is large.
Question 7.
Which noble gases can form chemical compound ?
Answer:
Kr and Xe can form compounds in extremely specific conditions.
Question 8.
Fluorine is strong oxidizing agent as compared to chlorine. Why ?
Answer:
Because of following points, fluorine is strong oxidizing agent:
- The size of F atom is smaller than Cl atom.
- Electronegativity of fluorine is higher than Cl atom.
- Dissociation energy of fluorine is less than that of chlorine.
- E° value of fluorine is more than chlorine.
Question 9.
F2O is not considered to be the oxide of fluorine. Why ?
Answer:
Fluorine is the most electronegative element. Its electronegativity is higher than oxygen. In naming a compound less electronegative elements are first and then more electronegative elements are written. Therefore, F2O or OF2 is known as oxygen difluoride and not fluorine oxide.
Question 10.
Interhalogen compounds are more reactive than halogen. Why ?
Answer:
X — Y bonds of interhalogen compounds are more polar due to difference in the electronegativities of halogen and are generally weaker than X — Y bonds of pure halogen. Therefore, interhalogen compounds are more reactive than halogens.
Question 11.
Helium and Neon do not form compounds with Fluorine. Why ?
Answer:
Due to absence of cf-orbitals in the valence shell of He and Ne, their electrons cannot get excited into higher energy o’-sub-shells. Therefore, He and Ne do not form compounds with fluorine.
![]()
The p-Block Elements Short Answer Type Questions
Question 1.
H2O is liquid, while H2S is a gas at ordinary temperature. Explain.
Answer:
In H2O, oxygen is electronegative due to this intermolecular hydrogen bonding is found among H2O molecule and exists as liquid state.
On the other hand, sulphur is less electronegative due to this intermolecular hydrogen bonding is not found in H2S and it exists as gas
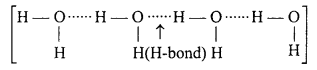
Question 2.
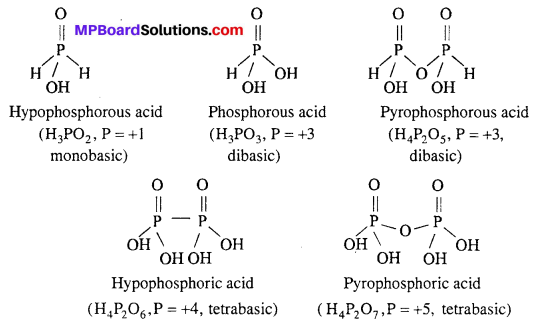
Write formula and structures of five oxy acids of phosphorus.
Answer:
Five oxy acids of phosphorous are :
(i) H3PO2 (Hypophosphorous acid)
(ii) H3PO3 (Phosphorous acid)
(iii) H4P2O5 (Pyrophosphorous acid)
(iv) H4P2O6 (Hypophosphoric acid)
(v) H4P2O7 (Pyrophosphoric acid).
Structures of oxy acids of phosphorus are :
Question 3.
Behaviour of oxygen is different from other elements of the group. Why ?
Answer:
Reason for abnormal behaviour of oxygen :
- Electronegativity is maximum.
- Ionisation energy is high.
- No any vacant d-orbitals present in valence shell.
- Atomic size is small.
- It forms strong hydrogen bonds.
Question 4.
What is the reason that oxygen is a gas whereas sulphur is a solid ?
Answer:
Oxygen forms diatomic O2 molecule. Different molecules of oxygen are tied with weak van der Waals forces, so oxygen is gas at normal temperature.
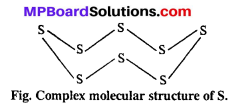
On the other hand, sulphur forms a complex molecular structure of 8 sulphur atoms. Molecular mass of S8 is very much and it is solid.
Question 5.
Water is neutral, whereas H2S is a weak acid. Why ?
Answer:
Due to the presence of strong H-bond in water, its molecules mutually associate due to which magnitude of its dissociation constant (ka) decreases. Therefore, water is neutral. Whereas in H2S due to lower electronegativity of S, hydrogen bond cannot be formed and size of sulphur atom is larger than oxygen atom due to which release of hydrogen in the form of proton is helpful. Thus, H2S is a weak acid.
Question 6.
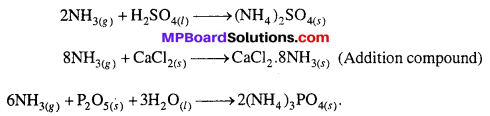
In the laboratory ammonia is dried by quick lime, why ?
Answer:
In the laboratory ammonium chloride is heated with caustic soda solution or with milk of lime in a hard, round bottom flask. Ammonia evolved is directly passed over quick lime and is collected in gas jars by downward displacement of air.
![]()
Aqueous NH3 is dried by passing it over quick lime (CaO). H2SO4, CaCl2 and P2O5, etc. cannot be used for drying NH3 because it reacts with them.
Question 7.
Write the difference between the bleaching action of SO2 and Cl2.
Or
Bleaching of flowers by Cl2 is permanent while bleaching by SO2 is temporary, why ?
Answer:
Bleaching action of SO2: Bleaching by SO2 is due to the process of reduction in the presence of moisture.
SO2 + 2H2O → H2SO4 + 2[H] (nascent)
The nascent hydrogen liberated in the reaction is responsible for bleaching flower but when the flower comes in contact with air. It gets oxidized and become coloured.
![]()
Bleaching action of Cl2: Bleaching by Cl2 is due to the process of oxidation in the presence of moisture.
Cl2 + H2O → 2HCl + [O] (nascent)
Coloured substance + [O] → Colourless
The oxygen liberated in the reaction is responsible for bleaching, colour bleached by Cl2 is permanent and original colour cannot be restored on exposure to air.
Question 8.
Write formula and structure of five Oxy acids of Sulphur.
Answer:
Oxy acids of Sulphur:
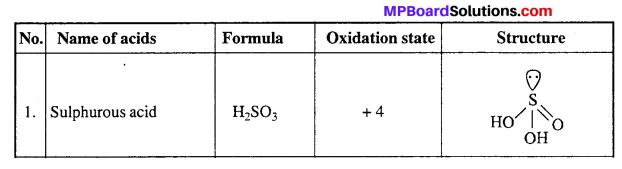
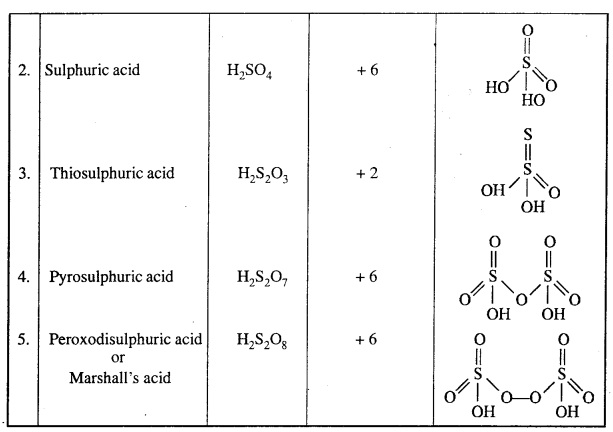
Question 9.
Write any four functions of Glover’s tower in lead chamber process for manufacturing of H2SO4.
Answer:
Functions of Glover’s tower :
(1) Oxide of nitrogen in nitrated sulphuric acid obtained by Glover’s tower, released
2NO + 2HSO4 + H2O → NO + NO2 + 2H2SO4
(2) By the temperature of gases, the water present in lead chamber evaporates and acid concentration increases upto 78%.
(3) In this tower SO2, NO2 and water vapour react to form H2SO4.
SO2 + NO2 + H2O → H2SO4 + NO
(4) Stones of Glover’s tower cool down and maintain the temperature 60-80°C.
Question 10.
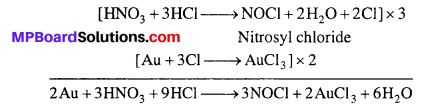
What is Aqua regia ? Write its uses.
Answer:
Aqua regia : Mixture of 1 part of cone. HNO3 and 3 part of cone. HCl.
Uses : It dissolves Au and Pt.
Question 11.
Contact process of manufacturing of H2SO4 is supposed to be more ad¬vance than lead chamber process. Why ?
Answer:
(i) The acid obtained by contact process is pure while the acid obtained by lead chamber process is impure.
(ii) The plant for lead chamber process requires large area while plant used in contact process occupies comparatively less area.
(iii) The catalyst used in contact process is platinised asbestos while in lead chamber process catalyst is gaseous (NO2 gas) and to maintain its flow regular is tedious.
(iv) In contact process concentrated sulphuric acid is obtained while in lead chamber process dilute acid is obtained.
(v) In contact process running of the plant is less costly while in lead chamber process running of the plant is costly.
Question 12.
The boiling point of NH3 is more than PH3. Why ?
Answer:
Ammonia molecules are associated together by hydrogen bond due to presence of N atom which is more electronegative than P atom of PH3 in which there is no hydrogen bond that is why boiling point of NH3 is high.
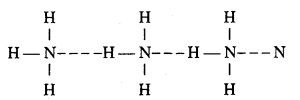
Question 13.
Draw labelled diagram of laboratory method of phosphine and give chemical equation.
Answer:
Phosphine is prepared in laboratory by heating white phosphorus with NaOH in an inert atmosphere of CO2 and oil gas. Phosphine produced is highly inflammable due to the presence of phosphorus dehydride as impurity. Vapour of phosphine form vortex noing of smoke in contact with air. Alcoholic KOH can be used in place of NaOH.
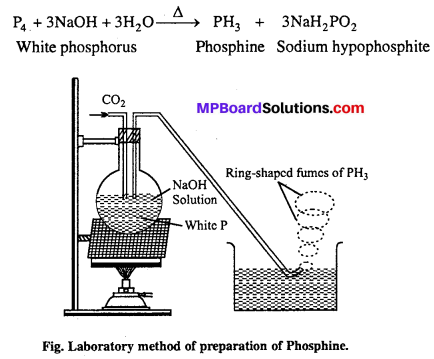
Question 14.
Nitrogen differs from its group 15 and show diagonal relationship with sulphur of group 16. Clarify the cause of similarity and difference.
Answer:
Nitrogen shows dissimilarities with other elements of that group due to following factors :
- Smaller atomic size.
- High electronegativity
- Tendency to form multiple bonds
- Non-availability of d-subshells.
Nitrogen resembles sulphur of group 16 in the following properties :
- Both are non-metal
- Both are bad conductor of electricity
- Both are electronegative element
- Both form covalent compound
- Both form stable and covalent hydride
- Oxide of both elements are soluble in water and form oxy acids
- Hydride of both elements dissolve in water.
Question 15.
Write the structure of Pyrophosphoric acid.
Answer:
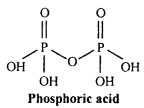
Oxidation number of phosphorus in pyrophosphoric acid is +5. It is tetrabasic acid. Pyro word is used for such acids which is obtained by the loss of one water molecule by heating two molecules. Its chemical formula is H4P2O7(P2O5.2H2O).
Question 16.
Oxygen exhibit -2 to +2 oxidation state while the other member of this group show +2, +4 and +6 oxidation state. Why ?
Answer:
The oxidation state of oxygen is -2, but in H2O2, O2, O2F2 and OF2 etc., the oxidation number of oxygen is -1, 0, +1 and +2. Oxygen is divalent because it contains two unpaired electrons. Its electronic configuration is 1s2,2s2, 2p2x, 2p1y 2p1z Therefore, there is no vacant nd orbital in oxygen. Thus, it does not have more than 2 valency whereas other element of this group contains vacant nd orbital. The electron from ns and np orbital can jump to nd orbital and gives rise to +2, +4 and +6 oxidation state.
Question 17.
Explain that molecular formula of oxygen is O2 whereas that of sulphur is Sg.
Answer:
Size of oxygen atom is small, thus it possesses the ability to form stable double bond with itself. Thus, its molecular formula is O2 where as due to large size of sulphur atom it does not form multiple bond, therefore it does not exist in the form of S2. Along with it due to high S-S bond energy it has catenation property more than oxygen. Therefore, sulphur exists in the form of S8 in which each sulphur atom is linked with other sulphur atoms by single covalent bonds and form puckered ring like structure.
Question 18.
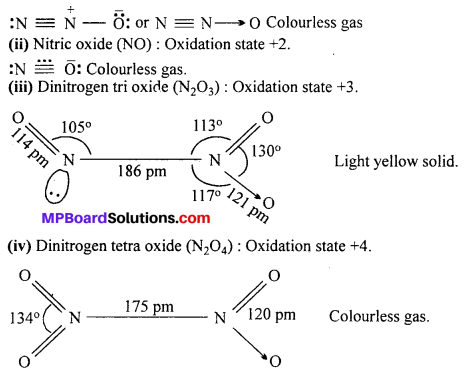
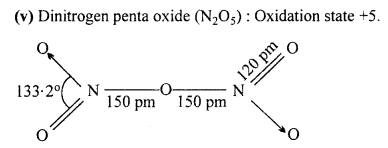
Write name, formula and oxidation state of oxides of nitrogen.
Answer:
Five oxides of Nitrogen are :
(i) Nitrous oxide (N2O): Oxidation state +1.
Question 19.
Explain Siemen-Halskey ozonizer process for the manufacture of ozone and draw labelled diagram.
Answer:
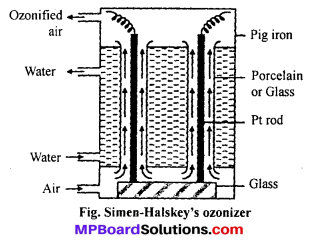
For commercial production of ozone Siemen-Halskey ozonizer is used. It consists of an iron box fitted with 6 to 8 glass cylinders. Each cylinder is 3 ft high and 10″ in width. The box is divided into three com-partments. Cold water flows through the central compartment for cooling. Each cylinder is fitted with an aluminium rod which are resting on insulating glass slab in the lower compartment. There is annular space between rod and sides of cylinder. A current of air flows through this annular space. The aluminium rods are subjected to high potential of 8,000 to 10,000 volts. The air which enters at bot¬tom escapes at the top and contains about 5% of ozone. If pure oxygen is used in place of oxygen then ozonized oxygen contains about 15% of ozone.
Question 20.
H2S is a stronger reducing agent than H2O. Why ? Give any three reasons.
Answer:
H2S is a stronger reducing agent than H2O because H2S can easily releases its hydrogen not H2O due to the following reasons :
(1) In H2O, H-bond is present which require extra energy to get released.
(2) Size of S is larger than oxygen.
(3) Electronegativity of oxygen is more than sulphur.
Question 21.
Why is sulphurous acid a reducing agent ?
Answer:
Because on sulphur atom of H2SO3 a non-bonding electron is still present. By losing this electron pair sulphur atom of H2SO3 can achieve higher oxidation state. Therefore, H2SO3 act as a reducing agent.
Question 22.
What is general electronic configuration of noble gases ? Write electronic configuration of noble gases.
Answer:
The general electronic configuration of noble gases is ns2np6. Where, n is the valence shell’s serial number.
Electronic configuration of noble gases are following :
Helium (He) → 1s2
Neon (Ne) → 1s2 2s2 2p6
Argon (Ar) → 1s2 2s2 2p6 3s2 3p6
Krypton (Kr) → 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xenon (Xe) → 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Radon (Rn) → 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6.
Question 23.
Explain with reason :
(a) HF is liquid while other hydrides of halogens are .gases at normal temperature.
(b) Fluorine does not form polyhalide.
Answer:
(a) The electronegativity of fluorine is maximum than other halogens. Therefore, HF molecule is conjugated by H-bond and the boiling point of HF is more to other halogen acids therefore HF is liquid while halides of other halogens are gases at room temperature.
H – F …. H – F …. H – F …. H – F
(b) The electronic configuration of fluorine is 1s2, 2s2, 2p5. Due to absence of d-orbital in its valency shell it does not show higher oxidation state and so it does not made polyhalide.
Question 24.
Explain, why :
(a) Noble gases are monoatomic ?
(b) Atomic radii of noble gases are largest ?
(c) Ionisation energy of noble gases are highest ?
Answer:
(a) There is no any unpaired electron in electronic configuration of noble gases. So these do not form chemical bonds and are monoatomic.
(b) Outermost shell of noble gases are completely filled so atomic radii of noble gases are maximum.
(c) All electrons in outermost orbit of noble gases are paired and energy required to make them unpaired is very high. Thus, noble gases have maximum ionisation energy in respective periods.
Question 25.
What do you mean by available chlorine ? Explain with reaction.
Answer:
When bleaching powder is treated with excess of dilute acid or carbon dioxide, the whole of chlorine present in the molecule is liberated. The amount of chlorine thus set free is called available chlorine.
CaOCl2 + 2HCl → CaCl2 + H2O + Cl2
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
CaOCl2 + CO2 → CaCO3 + Cl2
Amount of available chlorine in a good sample is 35-38%.
Question 26.
Write any two uses of helium gas.
Answer:
Uses of Helium : (i) It is used in filling air ships and balloons for weather study.
(ii) A mixture of 80% helium and 20% oxygen is used by divers for respiration.
![]()
Question 27.
Explain the structures of XeF2 and XeF4.
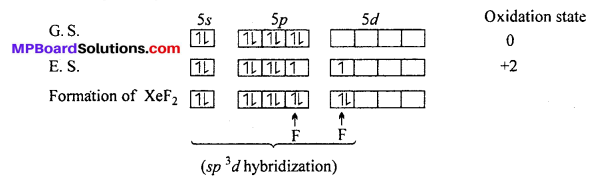
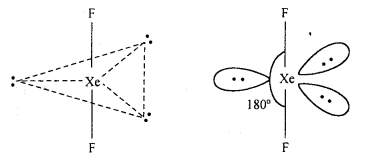
Answer:
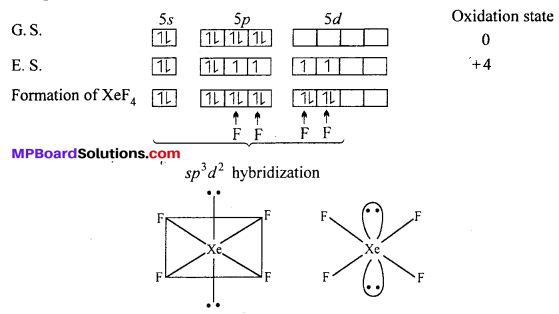
Structure of XeF2: In XeF2 molecule Xe atom is in sp3 d hybrid state hence, its structure is trigonal bipyramidal but its geometry is linear.
Structures of XeF4 : XeF4 formed under second excited state of Xe where two of sporbitals are unpaired and 4 unpaired electron develop. Being sp3 d2 hybridization involved, the structure is octahedral but due to presence of two lone pair of electron geometry of molecules get distorted and becomes square planar.
Question 28.
Fluorine always exhibit -1 oxidation state. Why ?
Answer:
Fluorine is the most electronegative element of periodic table. It always exhibit -1 oxidation state. It has no d-subshell in the outermost orbit. So it does not show any excited state.
Question 29.
Write the name, formula and oxidation number of any two oxy acids of chlorine.
Or,
Write formula structure and oxidation states of any two oxy acids of chlorine.
Answer:
Oxy acids of Chlorine :
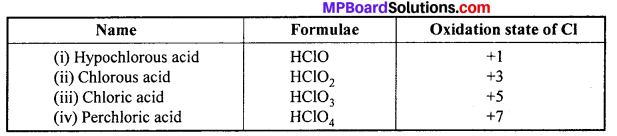
(i) Structure of HCIO : Central atom chlorine in CIO ion of HCIO is sp3 hybridized, due to which four hybrid orbitals are formed. Three of it contain lone electron pair and the fourth undergo overlapping with the orbital of oxygen forming sigma bond. The H+ geometry is linear.
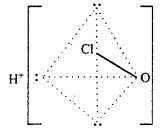
(ii) Structure of HClO3
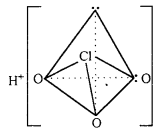
(iii) Structure of HClO4 : Its central atom Cl is sp3 hybridized. Thus, ClO4 ion is tetrahedral.
Question 30.
Explain bleaching action of bleaching powder.
Answer:
The cloth to be bleached is dipped in a solution of bleaching powder in water in a vat. From this vat, the cloth passes into a very dilute solution of hydrochloric acid. Here the cloth is bleached due to the liberation of chlorine by the action of acid on the bleaching agent. Some chlorine remains sticking to the fibre and is likely to damage it. In order to remove the traces of chlorine, the cloth is passed through a vat containing antichlor like sodium thiosulphate.
The cloth is then carried to another vessel where it is washed with excess of water. The washed cloth is then pressed with rollers, dried and ironed in the ironing cylinders and then wrapped in the form of a roll.
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
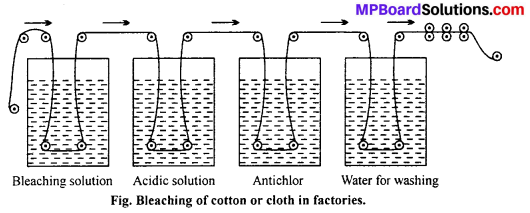
![]()
Question 31.
Noble gases are inert. Why ?
Answer:
Noble gases are inert due to following reasons :
- Noble gases are inert because octet of these gases are complete. It is the most stable state and do not have unpaired electrons.
- Ionisation energy of noble gases are high and electronegativity and electron affinity are zero. Therefore, these gases neither accept electron nor release electron.
Thus, noble gases are inert in nature.
Question 32.
Write any three anomalous behaviour of fluorine from other member of its group.
Answer:
Anomalous behaviour of fluorine are :
- Highest electronegativity of fluorine than other halogen. So, it can form F- ion and can displace other element.
- Due to smaller size, it forms strong covalent bond with other atom.
- Bond energy of fluorine is very low of about 158 kJ mol-1. Hence, it requires very less activation energy.
Question 33.
Xenon is a noble gas, then also it forms compound. Why ? Give structure of any two compounds.
Answer:
In 1962 Neil Bartlett observed that oxygen reacts with PtF6 and form O2[PtF6]. He thought that the first ionization energy of oxygen and xenon is nearly same, on this basis he treated Xe and PtF6 and got the first compound Xe+[PtF6].
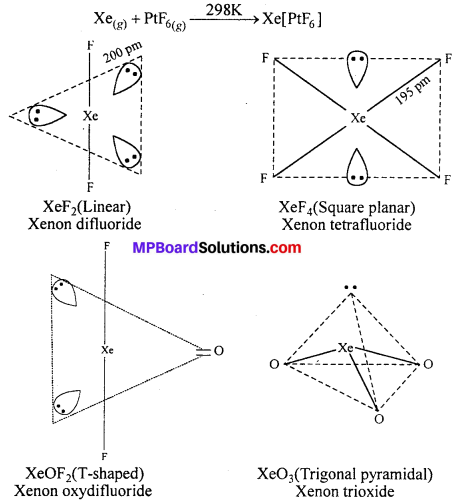
By direct action of Xe on PtF6, first compound of inert gases Xe+[PtF6]– was obtained. It is an orange-yellow crystalline solid.
Question 34.
Fluorine is strong oxidizing agent as compared to chlorine. Why ?
Answer:
Because of following points, fluorine is strong oxidizing agent:
- The size of F atom is smaller than Cl atom.
- Electronegativity of fluorine is higher than Cl atom.
- Dissociation energy of fluorine is less than that of chlorine.
- E° value of fluorine is more than chlorine.
Question 35.
Describe the laboratory preparation of chlorine.
Answer:
Laboratory preparation : HCl and MnO2 is taken in a round bottom flask. Cone. HCl is added from thistle funnel. The flask is heated slowly so that green yellow colour of Cl2 gas evolved.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
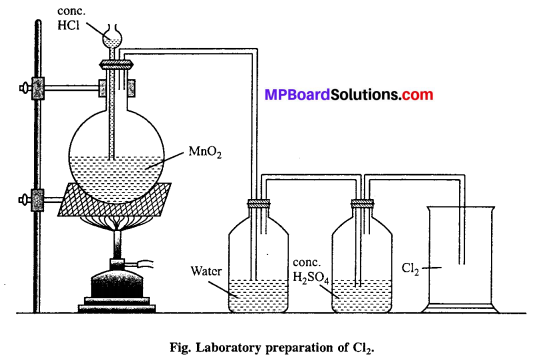
Question 36.
Write any five physical properties of noble gases.
Answer:
Five physical properties of noble gases :
- Atomic radii: Ionic radii of noble gases are corresponding to van der Waals’ radii. On moving downwards in the group, van der Waals’ radii increases.
- Ionisation energy : Due to stable electronic configuration, ionisation energy is very much. Value of ionisation energy decreases with increase in atomic number.
- Electron affinity : Due to stable electronic configuration ns2np6, noble gases do not have tendency to accept electron. Thus, electron affinity of these nearly zero.
- m. p. and b.p.: Due to weak attractive forces present in atoms m.p. and b.p. of these gases are low. Value of m.p. and b.p. increases on moving downward in the group.
- Liquification : Noble gases are not liquefied easily due to weak van der Waals’ forces.
Question 37.
Describe properties of hydrides of halogens under the following heads :
(i) Physical state
(ii) Thermal stability
(iii) Reducing property
(iv) Acidic strength
(v) Nature of bond.
Answer:
Properties of hydrides of hydrogen :
(i) Physical state: Hydrogen fluoride is a low boiling (292K) liquid while HC1, HBr and HI are gases. HF is liquid due to H-bonding.
(ii) Thermal stability : The order of thermal stability is
HF > HCl > HBr > HI
On moving down the group thermal stability decreases due to decrease in stability of bond with increase in size of halogen atom.
(iii) Reducing property : Reducing property increases down the group because stability of H – X bond decreases down the group.
HF < HCl < HBr < HI
HF does not have reducing property. HCl is weak reducing agent, HBr is strong reducing agent while HI is strongest reducing agent.
(iv) Acidic strength : Halogen hydrides are covalent compound in gaseous state. They ionize in gaseous medium and behave like acids. Their acidic strength is related to
stability of H – X bond strength. The decreasing order of acidic strength is
HF > HCI > HBr > HI
Dissociation energy of HF is highest hence, it does not ionises in aqueous solution.
Therefore, it is the weakest acid.
(v) Nature of bond: All halides are of covalent nature, but some possess ionic character also. Inonic character decreases from HF to HI.
Question 38.
Fluorine shows only -1 oxidation state while other halogen shows +3, +5 and +7 oxidation state in addition to -1 and +1, why ?
Answer:
F shows only +1 and -1 oxidation state, while other halogen i.e Cl, Br and I show +3, +5, +7 oxidation state in addition to +1, -1 because Cl, Br, and I halogen atom have vacant d-orbital on excited state they exhibit +3, +5 and +7 unpaired in d-orbital.
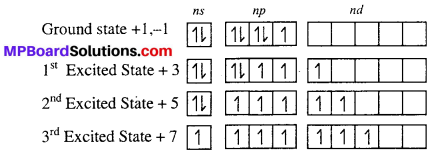
Therefore, except F other halogen show +3, +5 and +7 oxidation state in addition to -1 and +1.
Question 39.
Elements of group 17 (halogens) are coloured. Explain, why ?
Answer:
All elements of halogen group are coloured. Colour of these elements become deep with increase in atomic number.
Fluorine – Yellow
Bromine – Brown or deep red
Chlorine – Green-Yellow
Iodine – Violet.
Colour of halogens is due to absorption of visible light by molecules. Due to absorption of light outermost electrons excited to higher orbitals and elements are seen coloured. Being smaller atomic size, fluorine absorbs violet radiation of higher energy and are seen light yellow coloured while iodine atom having larger atomic size absorbs yellow radiation of lower energy and are seen violet coloured. For larger atomic size, radiation of less energy are required because electrons are far from the nucleus.
Question 40.
Give reason :
(a) Iodine exhibits some metallic property.
(b) Halogens are strongly oxidizing, Why ?
Answer:
(a) Ionization energy of iodine is small so it gives out one electron from its valence shell. In some reaction Iodine gives I+ ion so it has some metallic property.
I → I+ + e–
(b) Halogens are only one electron short to complete their octet and attaining stable structure. So halogens have strong tendency to accept electron. Thus, halogens are strong oxidising agents because of accepting electrons in reduction, oxidising power of halogens decreases from fluorine to iodine.
F2 > Cl2 > Br2 > I2
Question 41.
Fluorine is more active than other halogen. Explain, in three points.
Answer:
Fluorine is more active than other halogen because of:
- Bond energy of fluorine is minimum (158 kJ/mol), so F2 molecules require less activation energy for reaction.
- Size of fluorine atom is smaller than other halogen atoms, so the covalent bond formed with other atoms is stronger.
- Electronegativity of fluorine atom is highest and it forms F- ion easily. Due to more electronegativity it substituted other elements from their compounds.
![]()
The p-Block Elements Long Answer Type Questions
Question 1.
Give chemical reaction of ozone with
(i) K2MnO4
(ii) I2
(iii) Ag2O
(iv) CH2 = CH2 and
(v) PbS.
Answer:
(i) With K2MnO4 : Potassium permangnate is formed.
2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2
(ii) With I2 : Iodic acid is formed.
I2 + H2O + 5O3 → 2HIO3 + 5O2
(iii) With Ag2O : Silver is formed.
Ag2O + O3 → 2 Ag + 2O2
(iv) With CH2 = CH2: Ethene ozonide is formed.

(v) With PbS : PbSO4 is formed.
PbS + 4O3 → PbSO4 + 4O2
Question 2.
Explain Ostwald’s process of manufacture of nitric acid drawing labelled diagram.
Answer:
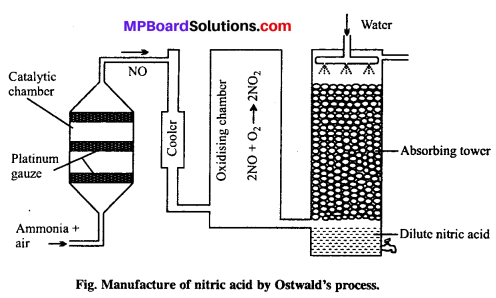
Principle of Ostwald’s process: The mixture of ammonia and air when passed over platinum gauze catalyst at 750 – 90OC, ammonia gets oxidized to pitric oxide.
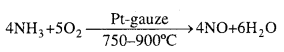
The reaction is exothermic and the heat liberated maintains the temperature of the catalyst. The nitric oxide is then oxidised to nitrogen dioxide by air which is cooled to 50”C and absorbed by water to produce nitric acid.
Question 3.
In which properties nitrogen exhibit difference with the other elements of that group and why ?
Answer:
Nitrogen shows dissimilarities with other elements of that group due to following factors :
- Smaller atomic size
- High electronegativity
- Tendency to form multiple ,bonds
- Non-availability of J-subshells.
Differences in properties :
- Nitrogen is less reactive gas while other members are reactive solids
- Nitrogen is diatomic while molecules of other elements are tetra-atomic (P4, AS4, Sb4)
- Nitrogen does not form complex compound while other elements of this group form complex compound due to presence of vacant d-orbitals
- Various oxides of nitrogen (N2O, NO, N2O3, N2O4 , N2O5) are known while other members do not form so many oxides
- NH3 is a liquid with high b.p. while other hydrides are gases
- Nitrogen shows catenation capability while it is absent in other elements
- Nitrogen does not show allotropism while other elements show allotropism
- Nitrogen does not form complex ions while other elements form complex
ions e.g., PF–4,SbI–6 etc.
Question 4.
Describe Brodie’s Ozonizer with diagram.
Answer:
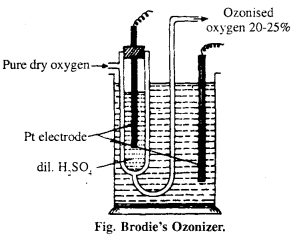
It is U-shaped glass tube, consists Pt wire with dil. H2SO4. The whole apparatus is put into glass utensil, outer utensil also contain H2SO4 and Pt electrode. Dry O2 is i passed through U-tube under the influence of high electric discharge oxygen gets converted to ozonised oxygen, the outcoming gas contains 20% of ozone.
Question 5.
Explain hydrides of nitrogen family on the following points :
1. Name and formula
2. Basic nature
3. Reducing property
4. Bond angle
5. Melting and boiling point.
Answer:

Basic nature: Basic nature decreases from NH3 to BiH3. Basic nature of these hydrides is due to the presence of lone pair of electron in the central atom.
Reducing nature: Reducing property increases in moving downwards from NH3 to BiH3. This is due to the decreasing stability of hydrides.
Bond angle: On going down the group the bond angle decreases due to decreasing electronegativity of central atom :

Melting point and boiling point: There is no regular trend of M.P. and B.P. of 15th group elements. M.P. increase from N to As whereas again decreases in Sb and Bi. B.P. increases continuously from N to Bi.
Question 6.
Explain hydrides of oxygen family on the following points :
1. Name and formula
2. Thermal stability
3. Reducing property
4. Acidic property
5. Covalent Character
Answer:

Thermal stability : Stability decrease with increase in molecular mass.

Reducing nature : The reducing power of hydrides of oxygen family increase in thermal stability.
Acidic nature : Water is neutral in nature while acidic nature increases from H2S to H2Te. This is due to increase in bond length in moving down the group which leads to increase hydrogen releasing tendency.
Covalent Character: Hydrogen has one electron in its outermost shell by which it can form covalent bond with the other elements of oxygen family and complete its outermost shell.
![]()
Question 7.
Explain manufacture of chlorine under the following heads :
(i) Labelled diagram of Nelson cell
(ii) Principle
(iii) Deacon’s process.
Answer:
(i) Nelson cell : In Nelson cell, graphite anode is suspended in a perforated cylindrical steel cathode provided with asbestos. Brine solution is taken in steel cathode. On electrolysis, chlorine gas is liberated at anode and is drawn Brine off through the outlet at the top. Na reacts with H2O at bottom to form NaOH and H2. Hydrogen escapes through the exit.
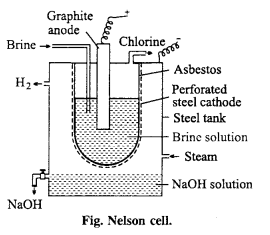
(ii) Principle: Nelson method or By electrolysis : Chlorine is manufactured by electrolysis of NaCl solution, chlorine is obtained as by-product.
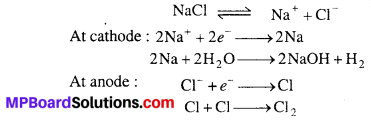
(iii) Deacon’s process : Previously chlorine was manufactured by Deacon’s process. In this process, HCl gas is heated with oxygen at 450°C in presence of CuCl2 catalyst.
Question 8.
Why elements of group 17 are called halogen ? Explain the following properties of halogen:
(i) Oxidation state
(ii) Electronegativity
(iii) Oxidizing property
(iv) Formation of bond with other element.
Answer:
Halogen means salt producer. The first four members of this group found in sea water. Therefore, the elements belonging to 17th group are called halogen.
Properties of halogen:
(i) Oxidation state : Common oxidation state of halogen is -1. Except fluorine the other halogen atom exhibit + 3, + 5, + 7 oxidation state.
F = -1
Cl = -1, +1, + 3, + 5, + 7
Br = -1, +1, +3, +5 .
I = -1, +1, +3, + 5, + 7.
Halogen have electronic configuration ns2np5 in their outermost energy level. So they have strong tendency to get or to share one electron for completion of octet, so they show -1 oxidation state when they combine with less electronegative elements.On combining with more electronegative elements they show +1 oxidation state. Oxidation state in HF, HC1 and HI is -1 and in ClF, BrF, IF, HClO, HBrO and HIO is + 1.
(ii) Electronegativity: The halogen have very high values of electronegativity. Fluorine is the most electronegative element with the electronegativity value 4.
(iii) Oxidizing property : Halogen are strong oxidizing agent due to high electron affinity, they have strong tendency to accept electron.
Decreasing order of oxidizing power is given as F2 > Cl2 > Br2 > I2.
(iv) Formation of bond with other element: Halogen form ionic bond with elec-tropositive element with the increase in the size of halogen atom, the tendency to form ionic bond decreases, e.g., AlF3 is ionic compound but AlCl3 is covalent compound. It forms covalent bond with non-metal.
Question 9.
Explain bleaching powder under the following points :
(i) Methods of preparation
(ii) Properties
(iii) Uses.
Answer:
(i) Methods of preparation: Bleaching powder is manufactured by the action of chlorine on dry slaked lime.
![]()
There are two plants for the manufacture of bleaching powder.
(a) Hasenclever plant : The plant consists of a number of horizontal cylinders provided with rotating shafts carrying blades.
Procedure: Slaked lime is added in the hopper and moved forward from one cylinder to the next by the revolving blades till it falls down. At the same time, dilute chlorine is passed up.
The formation of bleaching powder is based on the principle of counter currents as slaked lime and chlorine move in opposite direction to ensure complete conversion. Bleaching powder formed is collected in the receiver below.
(b) Modern method (Bachmann’s plant): Description of the plant: The plant consists of a vertical tower made of cast iron. It is provided with inlets for chlorine and hot air slightly above the base, a hopper at the top and an exit for the unused chlorine and air just below the top. There are a number of horizontal shelves at regular heights inside the tower.
Each shelf is provided with rotating rakes.
Dry slaked lime is fed into the chlorinating tower through the hopper at the top with the help of a suitable compressed air pumping arrangement. Slaked lime thus added, moves down with the help of rotating rakes. It meets the upgoing chlorine on the way and is con¬verted into bleaching powder which collects in the container, placed at the bottom. A current of hot air is passed to drive away unreacted chlorine.
(ii) Properties of bleaching powder:
(a) It is a pale yellow powder having strong smell of Cl2.
(b) It is soluble in cold water.
(c) In presence of a little cobalt chloride, it decomposes to liberate oxygen. On long standing it undergoes slow auto-oxidation and is converted into a mixture of calcium chlorate and calcium chloride.
(d) Reaction with insufficient acid: Bleaching powder is treated with small amount of dilute acid, it liberates hypochlorous acid which fumitus oxygen (nascent) and thus acts as oxidizing and bleaching agent.
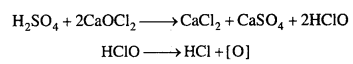
(e) With excess of acid : Bleaching powder reacts with excess of acid and liberates Cl2. The amount of Cl2 thus liberated is called “Available chlorine”.
![]()
(f) Reduction: Bleaching powder decomposes in presence of cobalt chloride liberating oxygen.
![]()
On keeping for long time it undergoes self oxidation forming calcium chlorate and calcium chloride.
![]()
(iii) Uses: (a) It is used as a disinfectant and germicide for the sterilization of drinking water.
(b) For the manufacture of chloroform.
(c) In textile and paper factories, for bleaching cotton, linen and wood pulp.
(d) For rendering wool unshrinkable.
Question 10.
What are interhalogen compounds ? How many types are they ? Give one example of each type with structure.
Or,
Explain hybridization in ABS and AB7 type of interhalogen compounds and draw their structures.
Answer:
Because of difference in the electronegativity value of halogen member it is possible to combine to form compound. When two different halogen combine together to form binary compound, then it is known as interhalogen compound.
There are four types of interhalogen compound, which has general formula XYn, where n = 1, 3, 5 or 7.
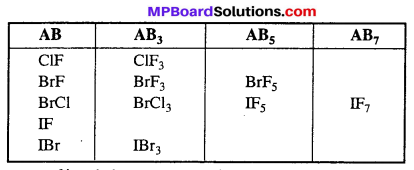
Structure of interhalogen compound :
1. AB Type : Like ClF, BrCl, IBr, ICl.
Their geometry is linear. In the ground state electronic configuration of chlorine it is clearly observed that there is one unpaired electron which forms covalent bond with other halogen atom.
2. AB3 Type : It is T-shaped. Like ClF3 molecule. Its central atom X shows sp3d hybridization.
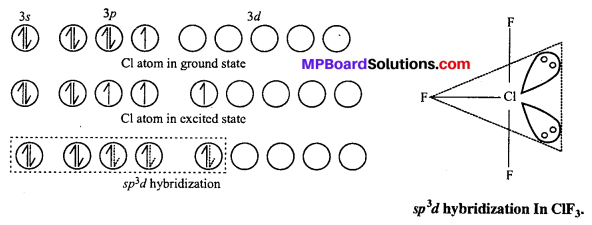
3. AB5 (IF5, BrF5) Type : These type of compounds show sp3d2 hybridization. Their structure is square pyramidal in which there is lone pair of electron at one place. Like in the figure formation of IF5 molecule is shown.
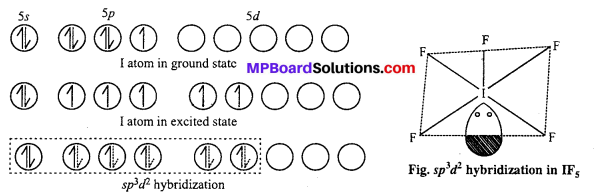
4. AB7 (IF7) Type : Its geometry is pentagonal bipyramidal which is formed by sp3d2 hybridization. Hybridization in IF7 molecule is sp3d3.
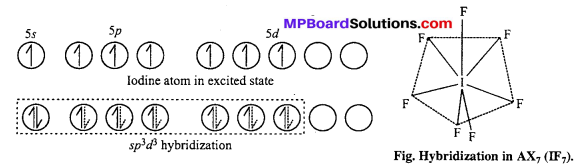
![]()
Question 11.
Write the uses of Noble gases.
Answer:
Uses of Noble Gases :
Uses of Helium : (i) It is used in filling air ships and balloons for weather study as it is light and non-inflammable.
(ii) Helium is used in research work for maintaining very low temperatures.
(iii) It is used in producing inert atmosphere, for food preservation and as filler in electric transformer.
(iv) Sea divers use mixture of helium and oxygen for deep sea diving. This is due to the fact that under high pressure helium is less soluble in blood as compared to nitrogen. If for breathing in deep sea diving air is used then more nitrogen will dissolve in blood under pressure and when the diver comes back at the surface of the sea, the solubility of nitrogen in blood decreases due to decrease in pressure and changes into nitrogen bubbles. This causes severe pain in diver’s body. It may also cause blisters and vomiting.
Uses of Neon : (i) Neon lights are used for commercial advertisements. It consists of a long tube fitted with electrodes at both ends. On filling the tube with neon gas and passing electric discharge of about 1000 volt potential, a bright red light is produced. Different colours can be obtained by mixing neon with other gases. For producing blue or green light neon is mixed with mercury vapours.
Uses of Argon : (i) Like helium, it is also used to create inert atmosphere in welding of aluminium ahd stainless steel.
(ii) It is filled in electric bulbs along with 25% nitrogen.
(iii) It is also used in radio valves.
(iv) Argon alone or its mixture with neon is used in tubes for producing lights of different colours.
Uses of Krypton and Xenon : (i) They are also used in electric bulbs in place of argon, but they are costly.
(ii) Both are used in flash photography.
(iii) Krypton is mainly used in producing fluorescence, incandescence and electric lights.
(iv) Krypton lamp is used as indicator at aeroplane terminals for landing of the aeroplane.
Uses of Radon: (i) In treatment of malignant growths (cancer) and non-healing wounds. Also, used in researches associated with radioactivity.
(ii) Used as a substitute for X-rays in radiology.
(iii) Radon is soluble in fatty compounds and recently a radon containing ointment has been manufactured for radiotherapy.