MP Board Class 12th Chemistry Solutions Chapter 4 Chemical Kinetics
Chemical Kinetics NCERT Intext Exercises
Question 1.
For the reaction R → P, the concentration of reactant changes from 0.03M to 0.02M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Answer:
For the reaction R → P
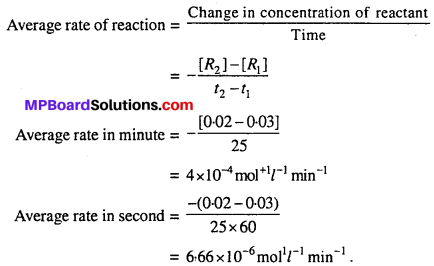
Question 2.
In a reaction, 2A → Products, the concentration of A decreases from 0.5 mol L-1 to 0.4 mol L-1 in 10 minutes. Calculate the rate during this interval ?
Answer:
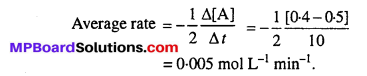
Question 3.
For a reaction, A + B → Product; the rate law is given by, r = k [A]1/2 [B]2. What is the order of the reaction ?
Answer:
Order of reaction = sum of powers of concentration of reactants.
Order of reaction = \(\frac { 1 }{ 2 } \) + 2 = \(\frac { 5 }{ 2 } \) = 2.5.
Question 4.
The conversion of molecules A; toy follows second order kinetics. If concetration of JC is increased to three times how will it affect the rate of formation of y2.
Answer:
For the reaction x → y
Rate of reaction (r) = k[x]2 …(1)
If concentration of x is increased three times, now
Rate of reaction (r)1 = K[3x]2 = k[9x2]
Dividing equation (2) by eq. (1)
\(\frac{r^{1}}{r}=\frac{k\left[9 x^{2}\right]}{k\left[x^{2}\right]}=9\)
Thus, rate of reactions will become 9 times.
Question 5.
A first order reaction has a rate constant 1.15 × 10-3 s-1. How long will 5g of this reactant take to reduce to 3g ?
Solution:
According to question,
![]()
Applying first order kinetic equation
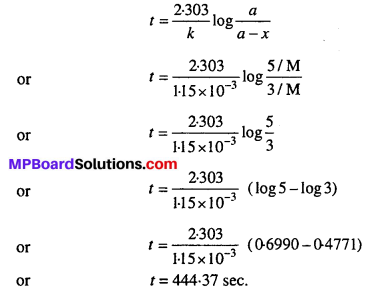
Question 6.
Time required to decompose SO2Cl2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
Solution:
For a first order reaction,
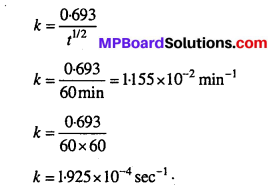
Question 7.
What is the effect of rate constant on temperature ? How can this effect of temperature be measured quantitatively ?
Answer:
Rate of reaction increases with increase in temperature and with 10°C rise in temperature its value becomes two times.
Arrhenius equation expresses the effect of temperature on constant
\(\mathrm{K}=\mathrm{A}_{e}^{-\mathrm{E}_{a} / \mathrm{RT}}\)
Where A is frequency factor and Efl is activation energy of the reaction.
Question 8.
The rate of the chemical reaction doubles for ah increase of 10K in absolute temperature from 298K. Calculate Ea.
Solution:
Given : k2 = 2k1, T1 = 298K, T2 = 308K, R = 8.314 JK-1 mol-1
We know that
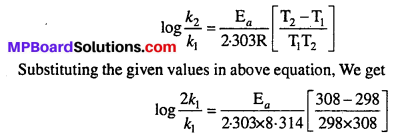
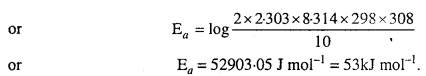
Question 9.
The activation energy for the reaction
2HI(g) → H2(g) + I2(g)
is 209.5kJ mol-1 at 581K. Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy ?
Solution:
According to question, Ea = 209.5kJ mol-1 = 209.5 × 103 J mol-1, T = 581K Fraction of molecules with energy equal to or greater than activation energy is given by
[R = 8.314J]
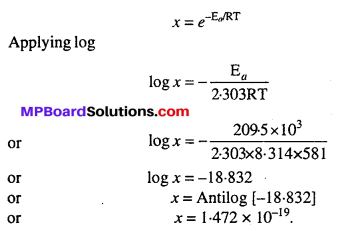
![]()
Chemical Kinetics NCERT TextBook Exercises
Question 1.
From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants.
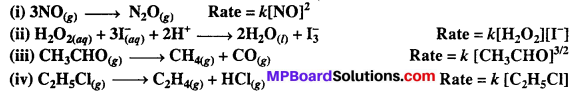
Solutions:

Question 2.
For the reaction :
2A + B → A2B
the rate= k [A][B]2 with k = 2.0 × 10-6 mol-2 L2 s-1. Calculate the initial rate of the reaction when [A] = 0.1 mol L-1, [B] = 0.2 mol L-1. Calculate the rate of reaction after [A] is reduced to 0.06 mol L-1
Solution:
Given, Rate = k [A][B]2
Initial rate = 2 × 10-6 × [0.1] × [0.2]2
= 8 × 10-9 mol L-1 s-1.
Decrease in concentration of A
i.e. ∆[A] = 0.1 – 0.06 = 0.04 mol L-1
Decrease in concentration of B
i.e. ∆[B] = \(\frac { 1 }{ 2 } \) × 0.04 = 002 mol L-1
Remaining concentration of B
= 0.2 – 0.02 = 0.18M
Rate = k[A] [B]2
= 2 × 10-6 × 0.06 × [0.18]2
= 3.89 × 10-9 mol L-1 s-1.
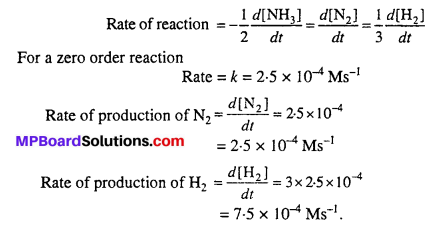
Question 3.
The decomposition of NH3 on platinum surface is zero order reaction. What are the rates of production of N2 and H2 if k = 2.5 × 10-4 mol-1 L s-1 ?
Solution:
2NH3 → N2 + 3H2
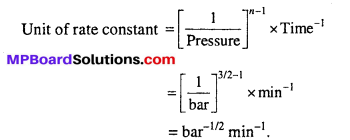
Question 4.
The decomposition of dimethyl ether leads to the formation of CH4, H2 and CO and the reaction rate is given by Rate = k [CH3OCH3]3/2. The rate of reaction is followed by increase in pressure in a closed vessel, so the rate can also be expressed in terms of the partial pressure of dimethyl ether, i.e.,
Rate = k(PCH3OCH3)3/2
If the pressure is measured in bar and time in minutes, then what are the units of rate and rate constants ?
Solution:
The rate law of reaction is
Rate = k [PCH3OCH3]3/2
Rate = Pressure change/Time change
Unit of rate = bar min-1
Question 5.
Mention the factors that affect the rate of a chemical reaction.
Answer:
Rate of reaction depends upon the following factors:
- Concentration of reactants
- Temperature
- Catalyst
- Nature of reactant etc.
Question 6.
A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is :
(i) doubled
(ii) reduced to half ?
Solution:
Let the reaction A → B is a second order reaction, for A
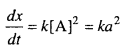
(i) When the concentration of [A] is doubled
A = 2 a
The new rate of reaction
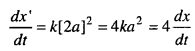
The rate of reaction will become four times when concentration is doubled.
(ii) When concentration of [A] is 1/2.
A = \(\frac { a }{ 2 } \)
So new rate of reaction

Therefore, the rate of the reaction could be reduced to l/4th.
Question 7.
What is the effect of temperature on the rate constant of a reaction ? How can this temperature effect on rate constant be represented quantitatively ?
Answer:
Rate constant of the reaction increases with temperature, it is independent whether the reaction is exothermic or endothermic. Arrhenius equation shows the dependence of rate constant on temperature.
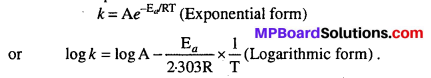
Question 8.
In a pseudo first order hydrolysis of ester in water, the following results were obtained:
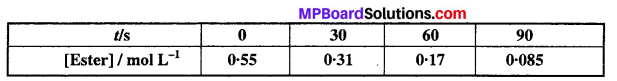
(i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(ii) Calculate the pseudo first order rate constant for the hydrolysis of ester.
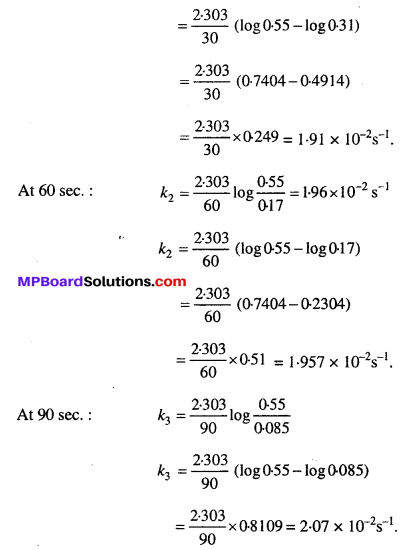
Solution:
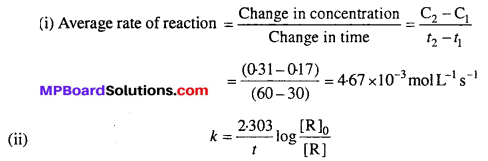
Where [R]0 = 0.55 M is initial concentration of ester (t = 0) and [R] is the concentration of ester at time ‘t’
![]()
Question 9.
A reaction is first order in A and second order in B.
(i) Write the differential rate equation.
(ii) How is the rate affected on increasing the concentration of B three times?
(iii) How is the rate affected when the concentrations of both A and B are doubled ?
Solution:
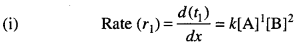
Let [A] = x and [B] = y
Rate (r1) = kxy2 ….(i)
(ii) When [B] = 3y
r2 = k x (3y)2 ….(ii)
On dividing equation (ii) by (i),
r2 = 9r1
i. e. rate increases 9 times.
(iii) When both [A] and [B] are doubled.
r3 = k (2x) (2y)2 ….(iii)
Dividing equation (iii) by (i),
r3 = 8r1
i.e. rate increases 8 times.
Question 10.
In a reaction between A and B, the initial rate of reaction (r0) was measured for different initial concentrations of A and B as given below:

What is the order of the reaction with respect to A and B ?
Solution:
Assuming that the order of reaction w.r.t. A is x and w.r.t. B is y.
Rate = k [A]x [B]y
Rate1 = 1(0.20)x (0.30)y = 5.07 × 10-5 ….(i)
Rate2 = k(0.20)x (0.10)y = 5.07 × 10-5 …..(ii)
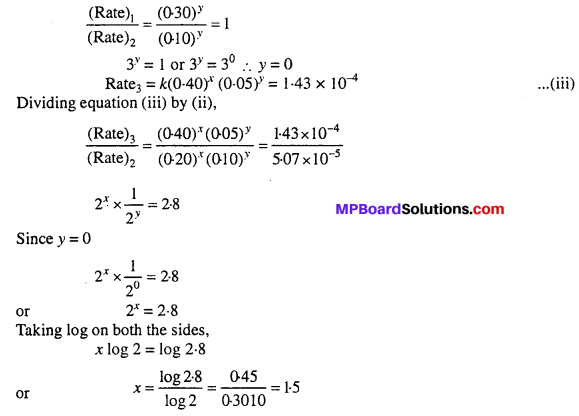
Order of reaction w.r.t A = 1.5
Order of reaction w.r.t B = 0.
![]()
Question 11.
The following results have been obtained during the kinetic studies of the reaction :
2A + B → C + D
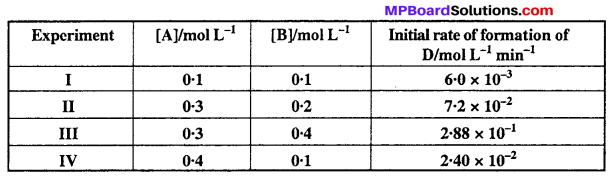
Determine the rate law and the rate constant for the reaction.
Solution:
Comparing experiments I and IV and substituting the values.
As concentration of ‘B’ remains constant therefore we get order w.r.t. ‘A’.
(Rate)1 = k (0.1)x (0.1)y = 6.0 × 10-3 ….(i)
(Rate)2 = k (0.4)x (0.1)y = 2.40 × 10-2 ….(ii)
Dividing equation (ii) by (i),
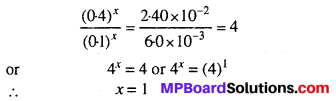
Similarly by comparing experiments II and III, we get the order w.r.t. ‘B’.
(Rate)2 = k (0.3)x (0.2)y = 7.2 × 10-2 ….(iii)
(Rate)3 = k (0.3)x (0.4)y = 2.88 × 10-1 …(iv)
Dividing equation (iv) by (iii),
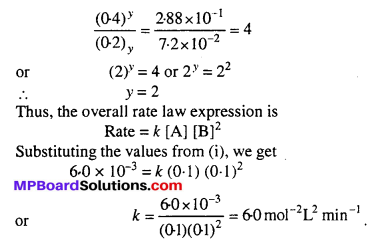
Question 12.
The reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table:
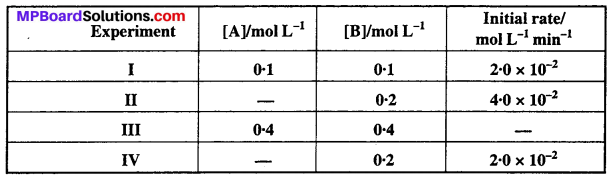
Solution:
Given that the reaction between A and B is first order w.r.t. A and zero order w.r.t. B.
∴ Rate = it [A]1 [B]0 but [B]0 = 1.
Rate = k [A]
From experiment I,
2 × 10-2 = k(0.1) ⇒ k = 0.2 min-1
From experiment II,
4 × 10-2 = 0.2 [A] ⇒ [A] = 0.2 min L-1
From experiment III,
Rate = (0.2) (0.4) = 0.08 mol L-1 min-1
From experiment IV,
2 × 10-2 = 0.2[A] ⇒ [A] = 0.1 mol L-1
Question 13.
Calculate the half-life of a first order reaction from their rate constants given below:
(i) 200 s-1
(ii) 2 min-1
(iii) 4 years-1.
Solution:
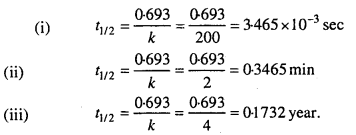
Question 14.
The half-life for radioactive decay of 14C is 5730 years. An archaeological artifact containing wood had only 80% of the 14C found in a living tree. Estimate the age of the sample.
Solution:
Radioactive decay follows first order kinetics,

Question 15.
The experimental data for decomposition of N2O5
[2N2Os → 4NO2 + O2]
In gas phase at 318K are given below:

(i) Plot [N2O5] against t.
(ii) Find the half-life period for the reaction.
(iii) Draw a graph between log [N2O5] and t.
(iv) What is the rate law?
(v) Calculate the half-life period from k and compare it with (ii).
Solution:
(i) The plot of [N2O5] versus time is shown below :
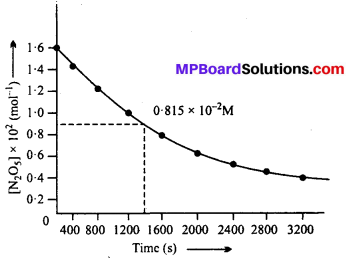
(ii) Initial concentration of N2O5 = 1.63 × 10-2 M
Half of initial concentration = 1.63 × 10-2 \(\frac { 1 }{ 2 } \) × = 0.815 × 10-2 M
Time corresponding to half of initial cone. (t1/2) from the plot = 1440 sec.
(iii) The plot of log [N2O5] Versus time :
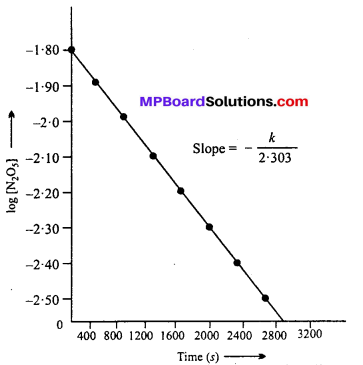
(iv) Since the graph between log [N2O5] vs time is a straight line, the reaction is of 1st order.
Rate = k [N2O5]

Question 16.
The rate constant for a first order reaction is 60s-1. How much time will it take to reduce the initial concentration of the reactant to its 1/16th value ?
Solution:

Question 17.
During nuclear explosion, one of the products is 90Sr with half-life of 28.1 years. If µg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years if it is not lost metabolically.
Solution:
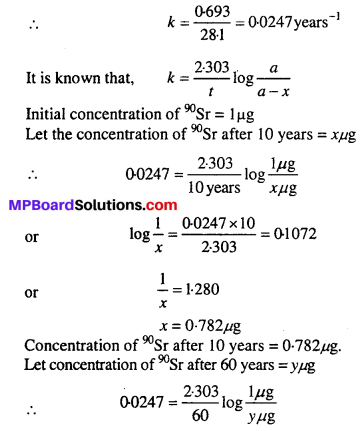
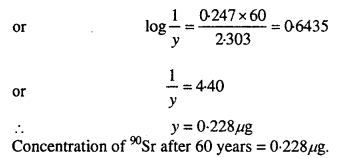
Question 18.
For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
Solution:
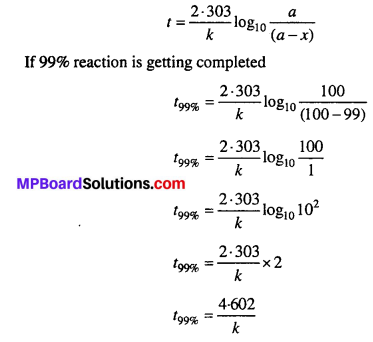
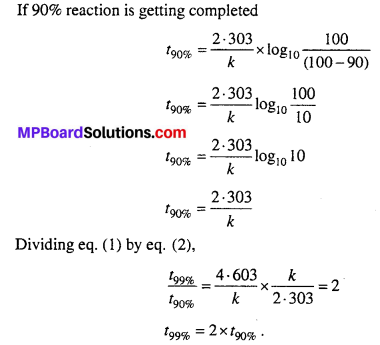
Question 19.
A first order reaction takes 40 min for 30% decomposition. Calculate t1/2.
Solution:
30% decomposition means that x = 30% of a = 0.30 a
As reaction is of first order
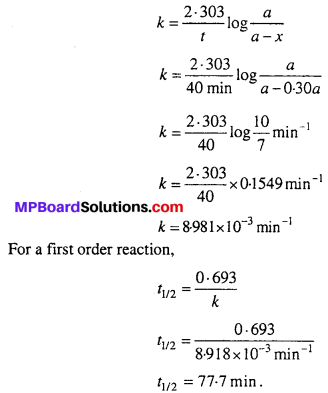
Question 20.
For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data are obtained :
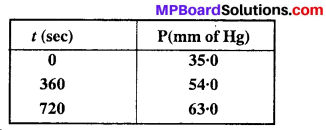
Calculate the rate constant.
Solution:
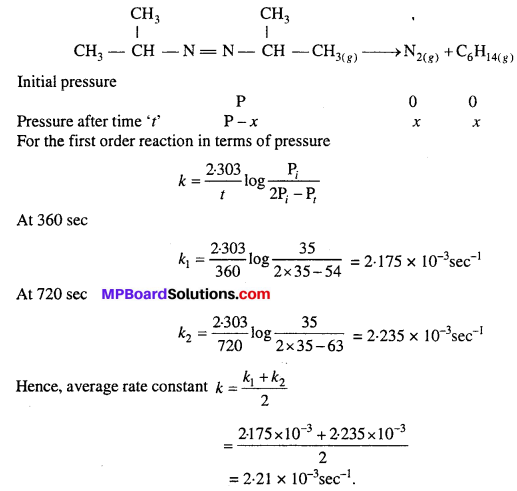
![]()
Question 21.
The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume.
SO2Cl2(g) → SO2(g) + cl2(g)

Calculate the rate of the reaction when total pressure is 0.65 atm.
Solution:
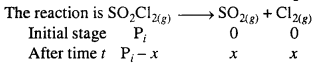
For the first order reaction in terms of pressure

Question 22.
The rate constant for the decomposition of N2O5 at various temperatures is given below:
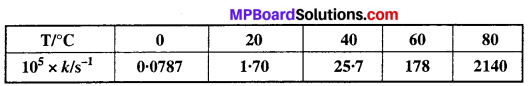
Draw a graph between In k and 1/T and calculate the values of A and Ea. Predict the rate constant at 30°C and 50°C.
Solution:
To draw the plot of log k versus 1/T, we can rewrite the given data as follows:
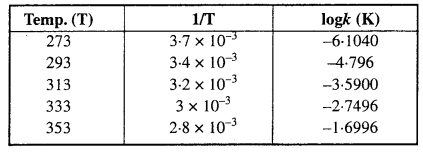
Draw the graph as shown in the ahead figure :
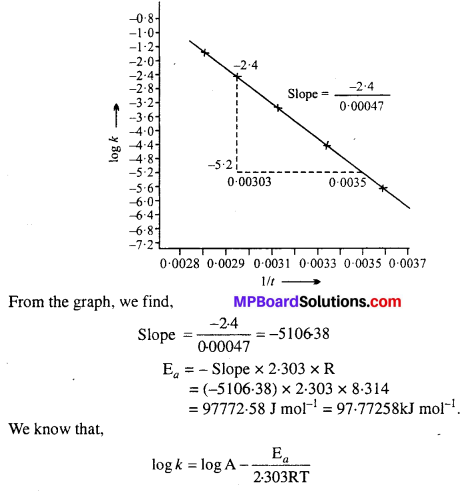
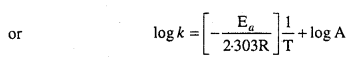
Compare it with y = mx + c
log A = Value of intercept on y axis i.e„ on logk axis
[y2 – y1 = -1 – (-7.2)] = (-1 + 7.2) = 6.2
log A = 6.2
A = Antilog 6.2
= 1.585 × 106 s-1
The value of rate constant k can be found from graph as follows :
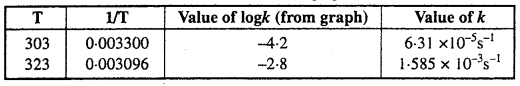
We can also calculate the value of k from the following formula :
![]()
Question 23.
The rate constant for the decomposition of hydrocarbons is 2.418 × 10-s s-1 at 546 K. If the energy of activation is 179-9 kj/mol, what will be the value of pre-exponential factor.
Solution:
According to the question, Rate constant (k) = 2.418 × 10-5 s-1, Temperature (T) = 546K, Activation energy (Ea) = 179.9 kJ mol-1
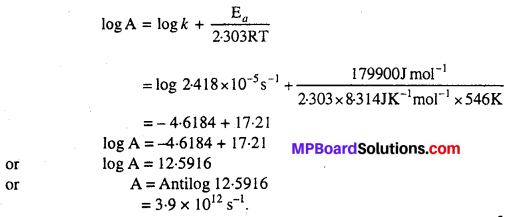
Question 24.
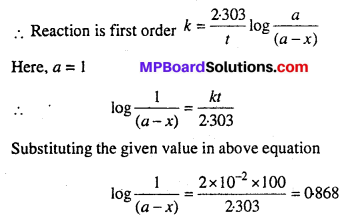
Consider a certain reaction A → Products, with k = 2.0 × 10-2 s-1. Calculate the concentration of A remaining after 100 s if the initial concentration of A is 1.0 mol L-1.
Solution:
Unit of k is s-1.
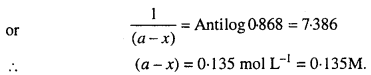
Question 25.
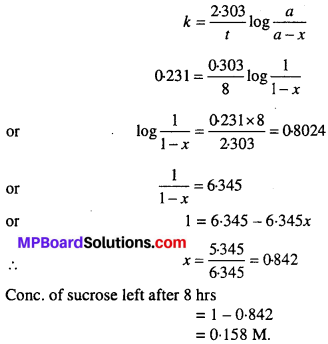
Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law, with t1/2 = 3.00 hours. What fraction of sample of sucrose remains after 8 hours?
Solution:
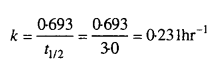
Let initial concentration of sucrose (a) = 1M
Concentration after 8hrs = (a – x) = (1 – x). Where ‘x’ is the amount of sucrose decomposed.
Question 26.
The decomposition of hydrocarbon follows the equation k = (4.5 × 1011 s-1) e– 28000 K/T Calculate Ea.
Solution:
k = (4.5 × 1011 s-1) e-28000K/T
Comparing the equation with Arrhenius equation
\(k=\mathrm{A} e^{-\mathrm{E}_{a} / \mathrm{RT}}\)

Question 27.
The rate constant for the first order decomposition of H2O2 is given by the following equation:
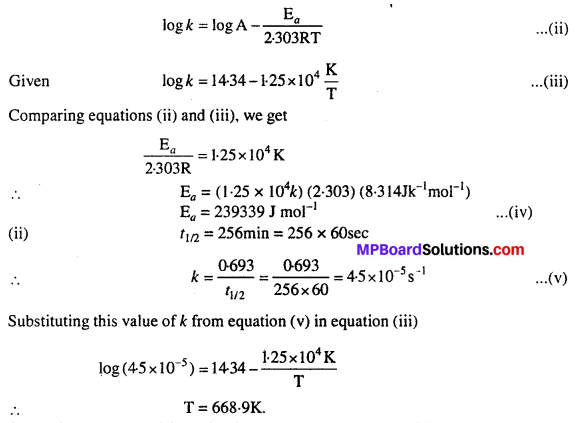
log k = 14.34 – 1.25 × 104 K/T
Calculate Ea for this reaction and at what temperature will its half-period be 256 minutes?
Solution:
(i) We know that
\(k=\mathrm{A} e^{-\mathrm{E}_{a} / \mathrm{RT}}\)
Taking logarithm on both sides of equation (i), we get
Question 28.
The decomposition of A into product has value of k as 4.5 × 103 s-1 at 10°C and energy of activation 60 kj mol-1. At what temperature would k be 1.5 × 104 s-1 ?
Solution:
Given k1 = 4.5 × 103 s-1, k2 = 1.5 × 104 s-1, T1 =10°C = 10 + 273 = 283K, Ea = 60kJ mol-1 = 60000J mol-1
Applying Arrhenius equation and substituting the values, we get
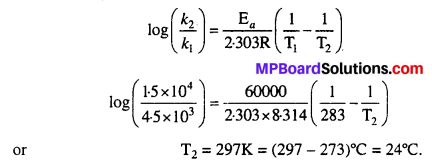
Question 29.
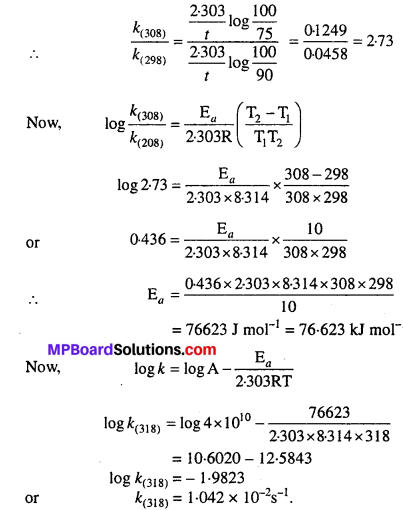
The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 × 1010 s-1. Calculate k at 318 K and Ea.
Solution:
For 10% completion of the reaction

Question 30.
The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
Solution:
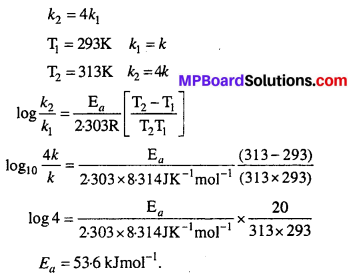
![]()
Chemical Kinetics Other Important Questions and Answers
Chemical Kinetics Objective Type Questions
Question 1.
Choose the correct answer :
Question 1.
For most of the reactions, the value of temperature coefficient lies in between:
(a) 1 and 3
(b) 2 and 3
(c) 1 and 4
(d) 2 and 4.
Question 2.
For first order reaction value of t1/2 is :

Question 3.
A first order reaction gets completed to 75% in 32 minutes. How much time would have been required for 50% completion :
(a) 24 minute
(b) 16 minute
(c) 8 minute
(d) 4 minute.
Question 4.
For reaction H2(g) + Br2(g) → 2HBr(g) the experimental values indicate that:
Rate of reaction = k [H2] [Br2]1/2 Molecularity and order of reaction is :
(a) 2, \(\frac { 3 }{ 2 } \)
(b) \(\frac { 3 }{ 2 } \),\(\frac { 3 }{ 2 } \)
(c) 1,1
(d) 1,\(\frac { 1 }{ 2 } \)
Question 5.
The reaction 2FeCl3 + SnCl2 → 2FeCl2 + SnCl4 is an example of:
(a) First order reaction
(b) Second order reaction
(c) Third order reaction
(d) None of these.
Question 6.
In the reaction 2A + B → A2B, the rate of consumption of reactant A is:
(a) Half of the consumption rate of B
(b) Equal to the consumption rate of B
(c) Twice to the consumption rate of B
(d) Equal to the rate of formation of A2B.
Question 7.
Hydrolysis of sucrose to glucose and fructose is :
C12H22O11 + H2O > C6H12O6 + C6H12O6
sucrose glucose fructose
an example of:
(a) First order reaction
(b) Second order reaction
(c) Third order reaction
(d) Zero order reaction.
Question 8.
At a given temperature the rate of reaction becomes slow when :
(a) Energy of activation becomes high
(b) Energy of activation becomes low
(c) Entropy changes
(d) Initial concentration of reactants remains constant.
Question 9.
Plants prepare starch in the process of:
(a) Flash photolysis
(b) Photolysis
(c) Photosynthesis
(d) None of these.
Question 10.
For first order reaction the specific reaction constant depends upon:
(a) Concentration of reactants
(b) Concentration of products
(c) Time
(d) Temperature.
Question 11.
In the reaction between A and B to form C, A represents first order and B rep¬resents second order. Rate equation will be written as :
(a) Rate = k [A]2 [B]
(b) Rate = k [A] [B]2
(c) Rate = k [A]1/2 [B]
(d) Rate = k [A] [B]1/2.
Question 12.
Molecularity of reaction of Inversion of sugar :
(a) 3
(b) 2
(c) 1
(d) 0.
Question 13.
Minimum energy required for molecules to react is called :
(a) Potential energy
(b) Kinetic energy
(c) Nuclear energy
(d) Activation energy.
Question 14.
2A + B → A2B, if concentration A is doubled and concentration of B is being half, the rate of reaction will:
(a) Increase 4 times
(b) Decrease 2 times
(c) Increase 2 times
(d) Remains unchanged.
Question 15.
Half life for second order reaction :
(a) Is proportional to initial concentration
(b) Does not depend upon initial concentration
(c) Inversely proportional to initial concentration
(d) Inversely proportional to square root of intial concentration.
Question 16.
The reaction 2H2O2 → 2H2O + O2, r = k[H2O2] is:
(a) Zero order reaction
(b) First order reaction
(c) Second order reaction
(d) Third order reaction.
Question 17.
Thermal decomposition of a compound is first order reaction. If a sample of compound decomposes 50% in 120 minutes, how much time it will take for 90% decomposition:
(a) About 240 minutes
(b) About 480 minutes
(c) About 450 minutes
(d) About 400 minutes.
Question 18.
Arrhenius equation is :
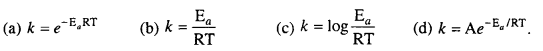
Question 19.
When temperature is raised, rate of reaction increases. This is due to :
(a) Decrease in activation energy
(b) Increase in number of collisions
(c) Decrease in number of active molecules
(d) Decrease in number of collisions.
Question 20.
Unit of rate constant of second order reaction is :
(a) mol-1 litre-1 second-1
(b) mol litre-1 second-1
(c) mol litre second
(d) mol-1 litre second-1.
Question 21.
Reaction
![]()
(a) Bimolecular and second order
(b) Unimolecular and first order
(c) Bimolecular and first order
(d) Bimolecular and zero order.
Question 22.
Unit of rate constant of first order reaction is :
(a) mol L-1 S-1
(b) mol-1 LS-1
(c) S-1
(d) mol L-1 S.
Question 23.
Time required for the completion of 90% reaction of first order is :
(a) 1-1 times that of half life
(b) 2-2 times that of half life
(c) 3-3 times that of half life
(d) 4-4 times that of half life.
Question 24.
The rate of chemical reaction depends upon :
(a) Active mass
(b) Atomic mass
(c) Equivalent weight
(d) Molecular mass.
Question 25.
Unit of reaction rate is :
(a) mol litre-1 second-1
(b) mol-1 litre second-1
(c) mol-1 litre-1 second
(d) mol litre second.
Answer:
1. (b), 2. (a), 3. (b), 4. (a), 5. (d), 6(b), 7. (a), 8. (a), 9. (c), 10. (d), 11. (b), 12. (c), 13. (d), 14. (d), 15. (c), 16. (b), 17. (d), 18. (d), 19. (b), 20. (d), 21. (c), 22. (c), 23. (c), 24. (a), 25. (a).
Question 2.
Fill in the blanks :
1. The rate of a reaction does not depends on the concentration of the reacting species, then the reaction is of …………………
2. Half life period of a radioactive element is 140 days. On taking 1 gm element initially the amount left after 560 days will be …………………
3. Fast reactions are completed in less than ………………… seconds.
4. In the mechanism of a reaction, the slowest step is called …………………
5. Study of fast reactions is done by …………………
6. Unit of rate constant for third order reaction is …………………
7. Difference in the minimum and maximum energy state of reactants is called …………………
8. Reactions which take place by the absorption of radiations are called …………………
9. The total number of molecules which participate in a reaction is called …………………
10. ………………… gives the idea about the mechanism of the reaction.
11. Hydrolysis of Ethyl acetate in acidic medium is an example of ………………… order reaction.
12. Molecularity is always a …………………
13. For the excitation of one mole reactant ………………… photons are required.
14. Rate of change in concentration of reactant at a specific instant of time is known as ………………… rate.
15. Rate of reaction is ………………… to the concentration of reactant.
16. cm-1 is the unit of …………………
Answer:
1. Zero
2. \(\frac { 1 }{ 16 } \) gm
3. 10-9
4. Rate determining step
5. Flash photolysis
6. mol-2 Litre2 second-1
7. Activation energy
8. Photochemical reactions
9. Molecularity
10. Order of reaction
11. Pseudo unimolecular
12. whole number
13. one mole
14. Instantaneous
15. Proportional
16. cell constant.
Question 3.
Match the following :
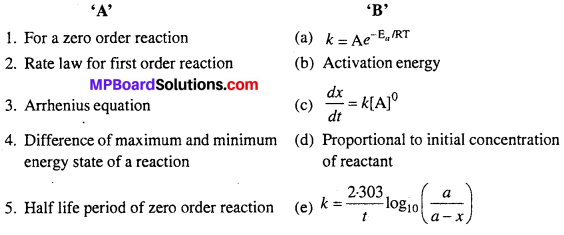

Answers:
- (c)
- (e)
- (a)
- (b)
- (d)
- (g)
- (f).
Question 4.
Answer in one word / sentence :
1. What is the relation between threshold energy and activation energy ?
2. What is the expression of rate constant for first order reaction ?
3. Write alternative form of Arrhenius equation.
4. If reaction A + B → C is a zero order reaction. Write rate law expression for it.
5. If rate equation for a reaction is Rate = k [NO2]2 [Cl2] then state the order of reaction with respect to Cl2 and order with respect to NO2 and total order of reaction.
6. Write rate equations on the basis of concentration of reactants and products for the following reactions.
2NO2 → 2NO + O2
7. What is rate determining step ?
8. Write an example of zero order reaction.
9. What is Quantum Efficiency ?
10. Write Arrhenius Equation.
11. Write the expression of half life period for first order reaction.
12. What is the effect of surface area of reactant on the rate of reaction ?
13. What is instantaneous rate ?
14. What is half-life period of a reaction ?
15. What is the unit of k for zero order reaction ?
16. Explain Threshold energy.
17. What are fast reactions ?
18. For a zero order reaction tm is proportional to what ?
Answer:
1. Activation energy = Threshold energy – Energy of molecules in normal state
2.
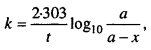
3.
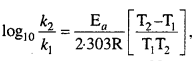
4.
![]()
5. Order with respect to cl2 = l, Order with respect to NO2 = 2, Thus, Total order = 1 + 2 = 3
6.
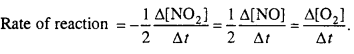
7. The slowest step is the rate determining step
8.
![]()
9. Quantum Efficiency

10.
![]()
11.
![]()
12. Larger surface area increases the rate of reaction
13. Rate of change in concentration of reactant or product at a specific instant of time is known as instantaneous rate
14. Half-life period of a reaction is the time in which concentration of reactant is reduced to one half of the initial concentration
15. Litre mol-1 sec-1
16. The minimum amount of energy which the reactant molecules should possess for effective collision
17. Reactions which gets completed in 10-9 second or even lesser time interval are called fast reactions
18. Initial concentration of reactant [A]
![]()
Chemical Kinetics Very Short Answer Type Questions
Question 1.
What is first order reaction ?
Answer:
If the rate of reaction depends on the first power of concentration of reactant then the order of reaction is said to be first order reaction.
Question 2.
What is Energy Barrier ?
Answer:
The minimum energy achieved by the reactant only after which it can be converted to product is known as energy barrier. Reactant molecules cannot form activated complex till they reach this height (activation energy) and cannot be converted to form product.
Question 3.
Explain the rate determining step.
Answer:
Some chemical reactions complete in one or more steps. Rate of reaction is determined by the slowest step which is known as rate determining step.
Question 4.
Write the characteristics of photochemical reactions. Any three.
Answer:
Characteristics :
- Absorption of magnetic radiations is necessary for these reactions.
- These reactions are unaffected by temperature but intensity of radiations affects them.
- Light is necessary for activating the reactants.
Question 5.
What are the applications of Arrhenius equation ? (Any two)
Answer:
- In calculating activation energy.
- In determining the rate constant at a temperature by the help of rate constant at another temperature of the reaction.
Question 6.
What is photochemical reaction ? Give an example.
Answer:
Such reactions which are induced by light or other electromagnetic radiations are known as photochemical reactions. Wavelength of these radiations is from 2000Å to 8000Å.

Question 7.
What is specific reaction rate ?
Answer:
Specific reaction rate of a reaction at a given temperature is equal to that rate of a reaction when concentration of each reactant is unity.
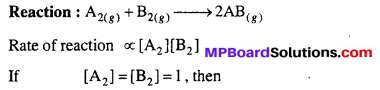
Rate of reaction = k where k = Specific reaction rate constant.
Question 8.
When is the average rate of the reaction equal to its instantaneous rate ?
Answer:
When value of time interval is nearly zero or when time by infinite form is minute then the average rate of reaction is comparable to its instantaneous rate.
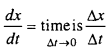
Question 9.
What are pseudo unimolecular reactions ?
Answer:
There are some reactions in which molecularity is more than one i.e. two or more molecules are present, but in the chemical reaction concentration of only one reactant molecule is changed and it is only responsible for rate of reaction. Thus, order is one. These reactions are called pseudo unimolecular reactions.
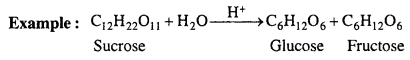
Above reaction is bimolecular but concentration of water does not affect the rate of reaction, thus, rate of reaction is proportional to the concentration of sucrose only.
Rate = k[C12H22O11]
Thus, inversion of sucrose is a first order reaction. It is known as pseudo unimoleciilar reaction.
Question 10.
What is temperature coefficient ?
Answer:
Generally, rate of any reaction increases due to increase in temperature. On increasing temperature by 10 C, velocity of reaction is increased up to 2-3 times. If velocity constant of a reaction is and it is increased to increasing temperature by 10 C, the ratio of these two constant is called temperature coefficient, i.e.,
![]()
Thus, at various time the ratio of rate of reaction which differ by 10°C is known as temperature coefficient.
![]()
Chemical Kinetics Short Answer Type Questions
Question 1.
Write down the expression representing the rate of reaction.
Answer:
Rate of reaction is the rate of change in concentration of reactant or concentration of product in unit time interval.
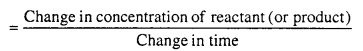
Unit of rate of reaction depend on the units of concentration and time. If concentration is represented in mole per litre and time in second, then unit of rate of reaction is mol per litre per second. If time is expressed in minute then unit of reaction rate is mole per litre per minute.
Question 2.
What is the meaning of instantaneous rate of reaction ?
Answer:
The rate of reaction does not remain constant during the whole time interval because rate of reaction depends upon the concentration of reactants. As the concentration of reactants decreases with time, the rate of reaction also decreases with time.
In order to express the reaction rate as accurately as possible, the instantaneous rate of reaction is expressed. For this the time interval (∆t) is taken as small as possible.

Where, d [A] is a change in concentration of reactant A.
Question 3.
What do you know about molecularity of any reaction ?
Answer:
Molecularity of Reaction : ‘Number of moles of reactant participating in elementary step of chemical reaction is called molecularity of the reaction.’
There are many reactions which proceed through the number of steps and each step being independent is called elementary step. The rate of reaction is determined by slowest step and it is known as rate determining step. Here molecularity can be defined as “Number of molecules/atoms or ions participating in rate determining step is called molecularity.”
Example : (i) Unimolecular reaction :
O3 → O2 + O
(ii) Bimolecular reaction:
NO + O3 → NO2 + O2
Question 4.
Differentiate molecularity and order of reaction.
Answer:
Differences between Molecularity and Order of reaction :
Molecularity:
- It is the total number of molecules which participate in the reaction.
- It is a theoretical concept.
- It is always a whole number.
- Its value is never zero.
- It does not provide any information about mechanism of reaction.
Order of reaction
- It is the number of molecules which participate in reaction and whose concentration is changed.
- Order of reaction is determined by experimentally.
- Fractional values are also possible.
- Zero value is possible.
- It provides information about mechanisms of reaction.
Question 5.
Write any four factors which affects rate of a chemical reaction.
Answer:
The rate of reaction depends upon the following factors :
(i) Concentration of reactants: At constant temperature, the rate of a reaction increases by increasing the concentration of the reactants.
(ii) Temperature of the system : If the concentration of the reactants are constant then the rate of reaction increases by increasing temperature. For 10 degree rise of temperature, the reaction rate becomes double or triple.
(iii) Presence of catalyst: Positive catalyst increases the rate of reaction and negative catalyst decreases the reaction rate.
(iv) Nature of the reactants: Nature of reactants also affect the reaction rate. In any chemical reaction some old bonds are broken and new bonds are formed. Thus, in case of more simple molecules the lesser is the number of bond breaking and the rate of reaction increase whereas in case of complex molecules more bonds are broken and rate decreases.
(v) Exposure to radiations: The rate of some reactions increases due to some special radiations.
Question 6.
Write characteristics of rate constant
Answer:
- At fixed temperature, the value of k is constant.
- For a particular reaction, k is independent of concentration but depends on temperature.
- The value of k is different for different reactions.
- It is a measure of intrinsic rate of reaction i. e., larger the value of k, faster will be the reaction and viceversa.
Question 7.
What do you understand by order of reaction ? Give example.
Answer:
Order of reaction : The order of a reaction is defined as the sum of all the powers to which concentration terms in the rate law are raised to express the observed rate of the reaction. Suppose there is a general reaction,
aA + bB + cC → product
For which the rate law is
Rate = – \(\frac { dx }{ dt } \) = k[A]p [B]q [C]r
Then the order of the reaction n = p + q + r, where, p, q and r are the orders with respect to individual reactants and overall order is the sum of the exponents i.e.,p + q + r.
When n = 1 the reaction is of first order, if n = 2 the reaction is of second order and so on.
For example : Decomposition of ammonium nitrite occurs as follows :
NH4NO2 → N2 + 2H2O
Rate of reaction = – \(\frac { dx }{ dt } \) = k[NH4NO2]
Thus, order of this reaction will be 1.
Question 8.
What do you mean by zero order reaction ? Give one example,
Answer:
In some reactions, rate does not depends, upon the concentration of reactants. This type of reactions are called zero order reaction.
Example : In contact of Au or Pt, the ammonia molecule dissociate and rate of this reaction is independent of concentration of ammonia.
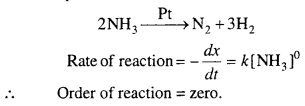
Question 9.
How does rate of any reaction depend on temperature ? Explain.
Answer:
Generally, rate of any reaction increases due to increase in temperature. On increasing temperature by 10°C, velocity of reaction is increased up to 2-3 times. If velocity constant of a reaction is and it is increased to increasing temperature by 10°C, the ratio of these two constant is called temperature coefficient, i.e.,
\(\frac{k_{t+10}}{k_{t}} \approx 2 \text { to } 3\)
Arrhenius provide the following relation for showing the effect of temperature on velocity constant.
i.e., \(k=\mathbf{A} \cdot e^{-\mathrm{E}_{\alpha} / \mathrm{RT}}\)
Question 10.
What is activation energy ?
Answer:
According to Arrhenius, any chemical reaction is only possible when reacting . molecules are activated with minimum energy which is called threshold energy. Kinetic energy of most of the molecules are less than this minimum energy. The excess energy which is required to activate reactant molecules, is called activation energy.
Activation energy can be determined by the use of Arrhenius equation.
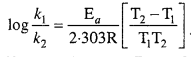
Question 11.
Write four differences between Rate of reaction and Rate constant.
Answer:
Differences between Rate of reaction and Rate constant:
Rate of reaction:
- It is expressed in terms of consumption of reactants or formation of product per unit time.
- It depends on concentration of reactant at particular moment.
- It generally decreases with the progress of reaction.
- Its unit is mol L-1 cm-1.
Rate constant:
- It is proportionality constant in differential form in rate law or rate equation.
- It is independent of concentration of reactant.
- It does not depend on the progress of reaction.
- It changes according to order of reaction.
Question 12.
Prove that half-life period of zero order reaction is proportional to initial concentration of reactant.
Answer:
If rate of reaction does not depend on the concentration of reactants, then it is known as zero order reaction.
For a zero order reaction the relation between rate constant and concentration is expressed by the following equation :
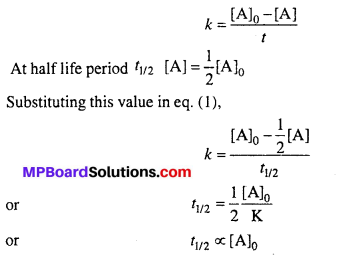
Thus, half-life period of zero order reaction is proportional to initial concentration of reactant.
Question 13.
Prove that half-life period is independent of the initial concentration for the first order reaction.
Answer:
Half-life period for the reaction is that period in which the initial concentration of reactant is reduced to half.
For first order reaction :
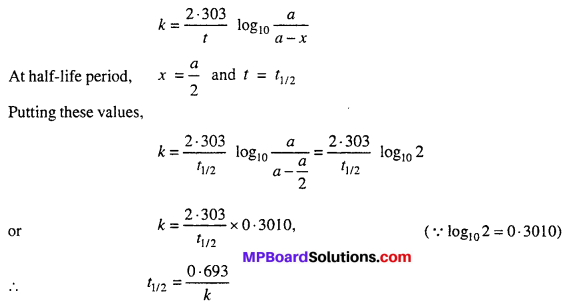
Thus, the half-life period is independent of the initial concentration for the first order reaction.
Question 14.
What is Integrated rate law method ? Write a note.
Answer:
Integrated rate equation for first order reaction is :
![]()
By this equation, order of reaction can be calculated. By knowing the initial concen¬tration (a) of reactant, concentration at a definite time (a – x) can be known.
This way, by substituting the values of t and (a – x), value of k is calculated. If value of k comes to be constant, the reaction is of first order. For, first order reaction, on plotting a graph between concentration log)0 (a – x) and t, a straight line is obtained. For other orders of reaction suitable equations are used and orders are determined.
Question 15.
A first order reaction is 90% complete in 40 minutes. Calculate its half- life period. (log 2 = 0.3010).
Solution:
Let initial cone, of reactant (a) = 100, t = 40 minutes
90% reaction gets completed in 40 minutes i.e.,
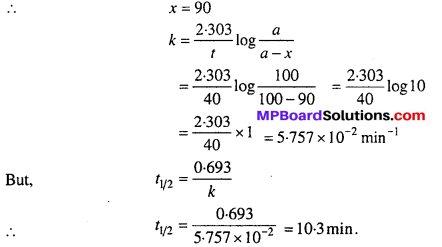
Question 16.
Show that time required for completing 99.9% of a first order reaction is 10 times of its half-life period.
Solution:
If initial concentration of reactant is a, then t = ? for x = 0.999 a
We know that for a first order reaction
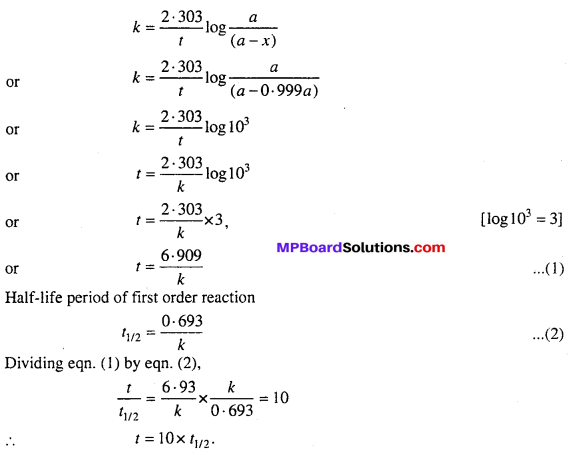
Question 17.
Write unit of rate constant k for the zero order, first order and second order reaction
Solution:
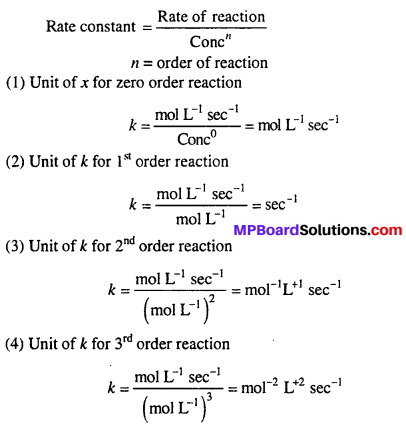
Question 18.
(1) 2N2Os 4NO2 + O2
(2) H22 + I2 → 2HI
Reaction (1) is first order reaction and (2) is second order reaction. Why ?
Answer:
(1) In reaction 2N2O5 → 4NO2 + O2, when graph is plotted between rate of reaction and then again graph is plotted between rate of reaction and [N2O5]2, it is shown that in the first graph straight line is obtained i.e.
rate ∝ [N2O5]
or rate = k[N2O5]
Therefore, 2N2O5 → 4NO2 + O2 is first order reaction.
(2) In reaction H2 + I2 → 2HI when graph is plotted between rate of reaction and (H2) (I2), it is seen that straight line is obtained.
Therefore rate ∝ [H2][I2]
Hence, this reaction is of 2nd order reaction.
![]()
Chemical Kinetics Long Answer Type Questions
Question 1.
Determine the expression for zero order reaction.
Answer:
Reactions in which rate of reaction does not depend on the concentration of the reactants are called zero order reactions. Consider a zero order reaction
A → B
Where, A and B are concentration of reactants and products. Since in this type of reaction, rate of reaction does not depend on the concentration of reactant therefore, rate of change in concentration of reactant remains constant.
Rate of reaction = Constant.
Let initial cone, of reactants be a moles /It and after time ‘t’ x moles are converted into product. Then cone, of A after time t will be (a – x) mol /It.
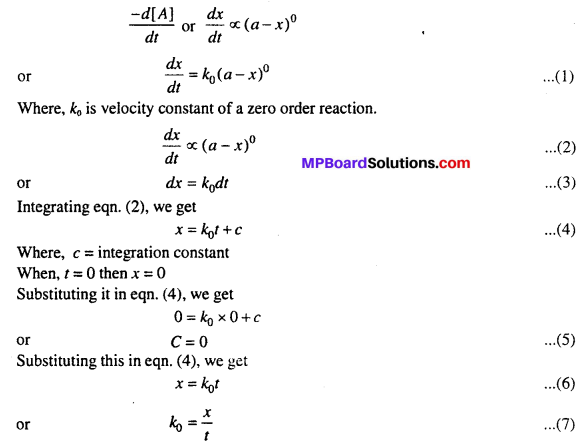
Thus, eqn. (7) is the velocity equation for zero order reaction.
Question 2.
Write a note on Arrhenius equation.
Answer:
Arrhenius equation : Arrhenius gave a relation between rate constant of reaction and temperature which is known as Arrhenius equation i.e.,
\(k=\mathbf{A} e^{-\Delta \mathbf{E}_{a} / \mathbf{R} \mathbf{T}}\)
Where, k = Rate constant of reaction, A = Frequency factor, Ea = Activation energy, R = Gas constant, T = Absolute temperature.
Taking logarithm both sides of above equation we get
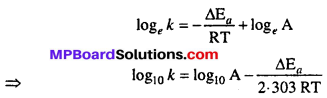
Ea and A can be determined by measuring rate constants of the reaction at two different temperature. Let k1 and k2 are the rate constant for the reaction at two temperatures T1 and T2 respectively. Then from eqn. (1)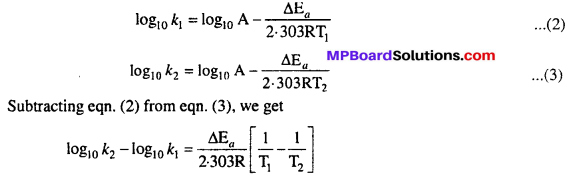
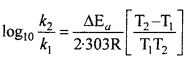
Application : Activation energy AEa may be calculated easily from this equation.
Question 3.
Write the Arrhenius equation in the form of equation of straight line. What will be the slope of a graph using this equation ? Calculate the activation energy for the decomposition in a decomposition reaction in which the value of slope obtained is – 9920 when log k is plotted against \(\frac { 1 }{ T } \).
Answer:
Arrhenius equation : Arrhenius gave a relation between rate constant of reac¬tion and temperature which is known as Arrhenius equation, i.e.,
\(k=\mathrm{A} e^{-\mathrm{E}_{a} / \mathrm{RT}}\)
Where, k = Rate constant of reaction, A = Frequency factor, Ea = Activation energy, R = Gas constant and T = Absolute temperature.
Taking logarithm both sides of above equation, we get
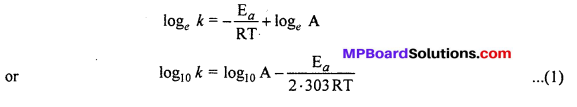
This is a straight line equation. If log10 A and \(\frac { 1 }{ T } \) is plotted at different temperatures, a straight line is obtained whose slope = \(\frac{-\mathrm{E}_{a}}{2 \cdot 303 \mathrm{R}}\) From the graph, value of slope = \(\frac{\mathrm{E}_{a}}{2 \cdot 303 \mathrm{R}}\) can be calculated and activation energy (Ea) can be calculated.
Alternatively, Ea and A can be determined by measuring rate constant of the reaction at two different temperatures. Let k1 and k2 are the rate constants for the reaction at two temperatures T1 and T2 respectively. Then, from eqn. (1),
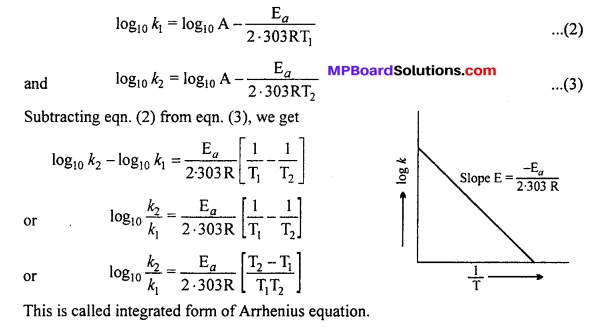
Calculation of activation energy :
Ea = -2.303 × slope × R = -2.303 × (-9920) × 1.986
∴ Ea = 45334 cal/gm/mol.
Question 4.
Derive an expression for velocity constant of first order reaction.
Answer:
The reaction in which velocity of reaction depends upon the concentration of one mole, are called first order reaction.
Let this reaction is
A → Product
Suppose intial concentration of A is a gram mole and after t second x mole consumed and remaining concentration is (a – x) gram mole.
So after t time, the rate of reaction will be proportional to (a – x)
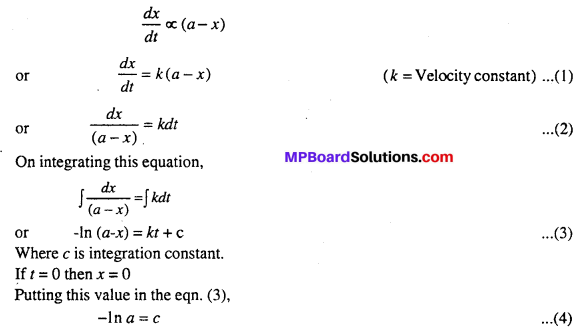
Putting the value of c from eqn. (4), into eqn. (3).
– ln(a – x) = kt – In a
or In a – ln(a – x) = kt
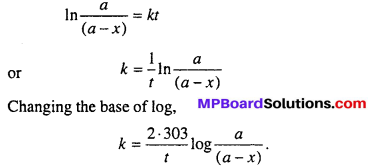
It is the desired expression for first order of reaction.