In this article, we will share MP Board Class 11th Chemistry Solutions Chapter 13 Hydrocarbons Pdf, These solutions are solved by subject experts from the latest edition books.
MP Board Class 11th Chemistry Solutions Chapter 13 Hydrocarbons
MP Board Class 11 Chemistry Hydrocarbons Textbook Questions and Answers
Question 1.
How do you account for formation of ethane during chlorination of methane?
Solution.
Chlorination of methane takes place through a free radical chain mechanism as given below:
(a) Chain initiation:

(b) Chain propagation:
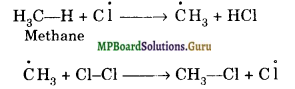
(c) Chain termination:
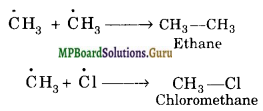
From the above mechanism, it is evident that during chain propagation step, ![]() free radicals are produced. In the chain termination step, the two
free radicals are produced. In the chain termination step, the two ![]() free radicals may combine together to form methane (CH3-CH3) molecule.
free radicals may combine together to form methane (CH3-CH3) molecule.
Question 2.
Write IUPAC names of the following compounds:
(a) CH3 CH = C(CH3)2
(b) CH2 = CH-C≡C-CH3


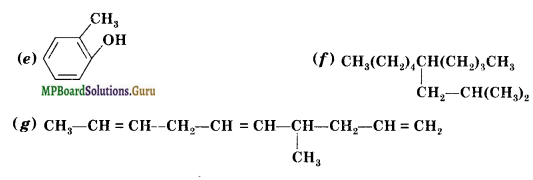
Solution:
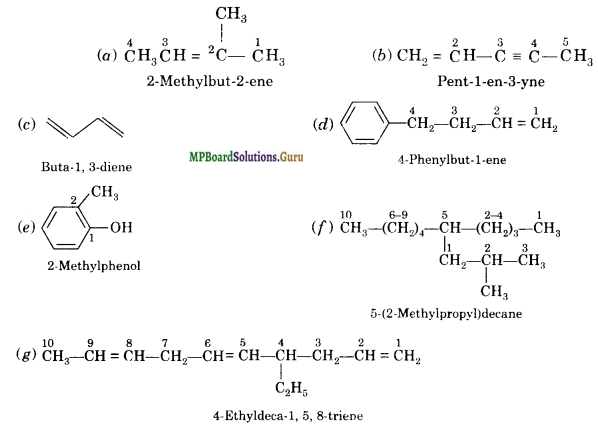
Question 3.
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated: I
(a) C4H8 (one double bond)
(b) C5H8 (one triple bond)
Solution.
(a) Isomers of C4H8 having one double bond are:
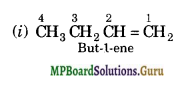
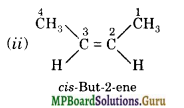
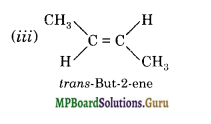
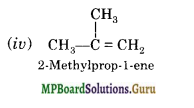
(b)
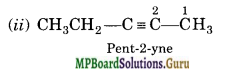
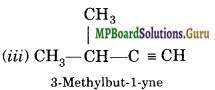
Question 4.
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) pent-2-ene
(ii) 3, 4-Dimethylhept-3-ene
(iii) 2-Etylbut -1-ene
(iv) 1-Phenylbut-l-ene.
Solution:
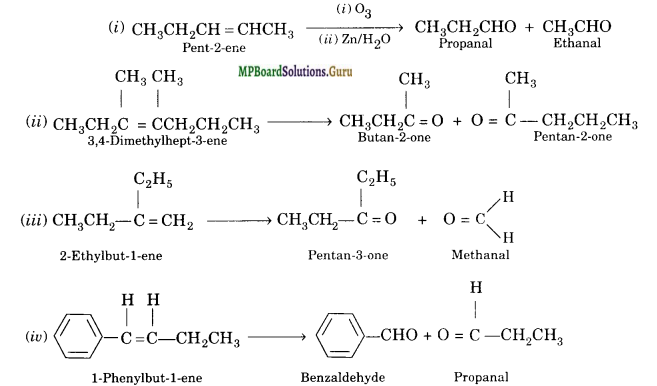
Question 5.
An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one. Write the structure and IUPAC name of ‘A’.
Solution.
Write the structure of the products side by side with their oxygen atoms pointing towards each other.
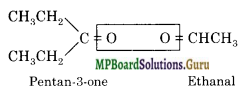
Remove the oxygen atoms and join the two ends by a double bond, the structure of the alkene ‘A’ is :
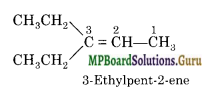
![]()
Question 6.
An alkene ‘A’ contains three C-C, eight C-H σ bonds and one C-C π-bond, ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 a.m.u. Write the IUPAC name of an.
Solution.
Let the aldehyde with molecular mass 4 amu be CnH2n +1. CHO
The molecular mass of CnH2n +1 CHO would be = 12n + 2n + 1 + 12 + 1 + 16 = 14n + 30
But molecular mass = 44 (given)
∴ 14n + 30 = 44
14 n = 14 or n = 1
Thus, the molecular formula of the aldehyde is CH3CHO. The alkene which on ozonolysis gives 2 moles of CH3CHO for each mole of alkene is :
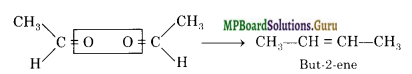
But-2-ene has three C-C sigma bonds, eight C-H sigma bonds and one C-C re bond.
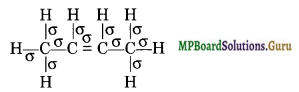
Thus, the compound ‘A’ is but-2-ene.
Question 7.
Propanal and pentan-3-one are the ozonolysis products of an alkene. What is the structural formula of the alkene?
Solution.
Write the structures of propanal and pentan-3-one with their oxygen atoms facing each other, we have,

Remove oxygen atoms and join the two carbon atoms by a double bond,
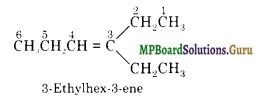
Question 8.
Write chemical equations for the combustion reaction of the following hydrocarbons:
(i) Butane
(ii) Pentene
(iii) Hexyne
(iv) Toluene.
Solution.
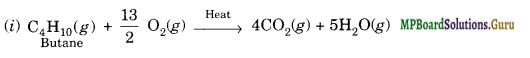
![]()
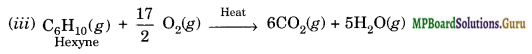
![]()
Question 9.
Draw the cis and trails structures of hex-2-ene. Which isomer will have higher b.p. and why?
Solution.
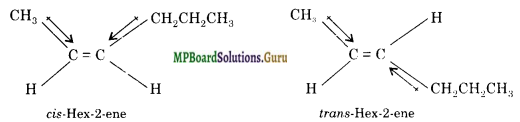
cts-Hex-2-ene has higher boiling point because it has higher dipole moment and hence stronger intermolecular attractive forces. In tran.s-hex-2-ene, the individual bond dipoles oppose each other thus making it almost non-polar.
Question 10.
Why is benzene extraordinarily stable though it contains three double bonds?
Solution.
Write the structure of the products side by side with their oxygen atoms pointing towards each other.

Remove the oxygen atoms and join the two ends by a double bond, the structure of the alkene ‘A’ is :

![]()
Question 11.
What are the necessary conditions for any compound to show aromaticity?
Solution.
The conditions for a compound to show aromaticity are:
(i) The molecule must be cyclic
(ii) It must have a conjugated system of (4n + 2) π-electrons
(iii) The molecule must be planar so that ample delocalization of π-electrons can take place.
Question 12.
Explain why the following systems are not aromatic?
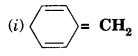
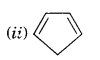
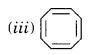
Solution.
 It does not contain all the six it-electrons in the ring. Therefore, it is not an aromatic compound.
It does not contain all the six it-electrons in the ring. Therefore, it is not an aromatic compound.
 It contains only four electrons, therefore, the system is not aromatic because it does not contain (4n + 2) π-electrons.
It contains only four electrons, therefore, the system is not aromatic because it does not contain (4n + 2) π-electrons.
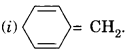
Cyclooctatetraene is a conjugated system having 8 π-electrons.
Therefore, the molecule is not aromatic since it does not contain (4n + 2) π-electrons.
Question 13.
How will you convert benzene into
(i) p-Nitrobromobenzene
(ii) m-Nitrochlorobenzene
(iii) p-Nitrotoluene Solution.
(iv) Acetophenone?
Solution:
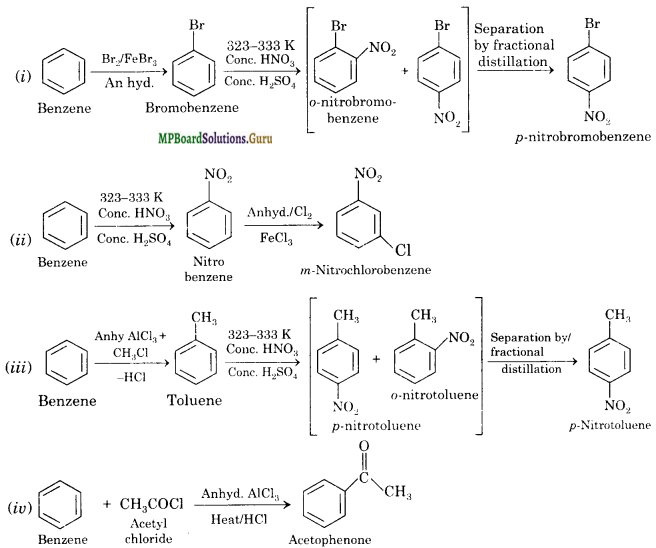
Question 14.
In the alkane, H3CCH2C(CH3)2 CH2CH(CH3)2, identify 1°, 2°, 3°, carbon atoms and give the total number of H atoms bonded to each one of these.
Solution.
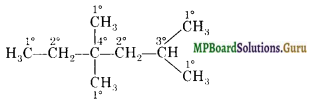
1° Carbon atoms = 5
Hydrogen atoms attached to 1° carbon atoms = 15 2° Carbon atoms = 2
Hydrogen atoms attached to 2° carbon atoms = 4 3° Carbon atom = 1
Hydrogen atoms attached to 3° carbon atom = 1.
Question 15.
What effect does branching of an alkane chain has on its melting point?
Solution.
Melting point of a compound depends not only upon size of the molecule but also upon the packing of the molecules in the crystal lattice. Generally, the branching in alkane results in less efficient packing and hence more the branching in the alkane, lower is the melting point. However, if the branching leads to a more compact structure, which can pack more closely in solid state, the melting point increases.
For example, among isomeric pentanes, neo-pentane has the highest melting point (because its molecules being almost spherical pack more closely in crystal lattice) while 2-methyl butane has the lowest (because its molecules pack least efficiently due to side chain). Thus, branching may increase or decrease the melting point.
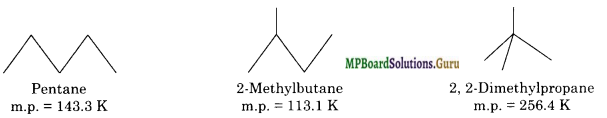
![]()
Question 16.
Addition of HBr to propene gives 2-bromopropane, while in presence of benzoyl peroxide, the same reaction gives 1-bromopropane. Explain and give mechanism.
Solution.
See mechanism of addition of halogen acids to alkenes and mechanism of anti-Markownikov addition in the addition reactions of alkenes.
Question 17.
Write down the products of ozonolysis of 1,2-di metliyl benzene (o-xylene). How does the result support Kekule’s structure for benzene?
Solution.
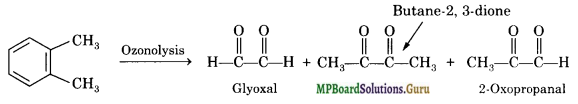
The products of ozonolysis are glyoxal, 2, 3-butanedione and 2-oxopropanal. The products of ozonolysis suggest three double bonds in the ring. This also suggests that the positions of double bonds as shown in I and as shown in II. This supports Kekule’s structure of
benzene.

The structure I on ozonolysis is expected to yield glyoxal and 2-oxopropanal while structure II is expected to yield glyoxal and 2, 3-butanedione.
Question 18.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Solution.
The decreasing order of acidic behaviour is ethyne > benzene > n-hexane. The C-H bonds in ethyne, benzene and hexane are formed by sp-s, sp2-s and sp3-s overlap respectively. Since sp-hybridized carbon is more electronegative then sp2-hybridized carbon which in turn is more electronegative than sp3-hybridized carbon, Therefore, the polarity of C-H bond is in the order : ethyne > benzene > n-hexane.
Consequently, the acidity is in the order: ethyne > benzene > hexane.
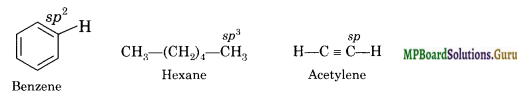
Question 19.
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Solution.
Benzene molecule has two n cloud rings, one above and the other below the plane of atoms. Therefore, it is likely to be attacked by electrophiles which subsequently brings about substitution.
The nucleophiles would be repelled by the π-electron rings and hence benzene reacts with nucleophiles with difficulty.
Question 20.
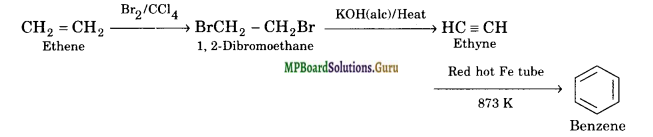
How will you convert the following compounds into benzene?
(i) Ethyne
(ii) Ethene
(iii) Hexane.
Solution.
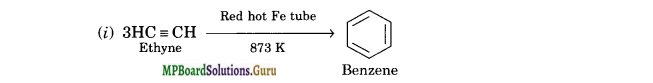
(ii) Ethene is first converted into ethyne and then to benzene.
(iii) When vapours of hexane are passed over heated catalyst consisting of Cr2O3, Mo2O3 and V2O5 at 773 K under 10-20 atm pressure, cyclization and aromatization occurs simultaneously to yield benzene.
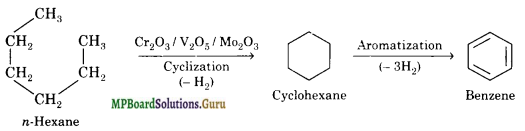
Question 21.
Write structures of all the alkenes which on hydrogenation give 2-methyl butane.
Solution.
Alkenes having the following carbon chain skeleton would give 2-methyl butane on hydrogenation

Following alkenes are possible for above carbon chain skeleton.
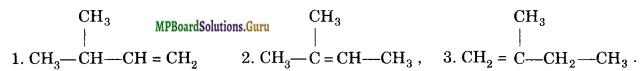
Question 22.
Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile, E+.
(i) Chlorobenzene, 2, 4-dinitrochlorobenzene, p-nitrochlorobenzene
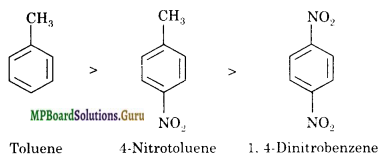
(ii) Toluene, p-H3C-C6H4-NO2 , p-O2N-C6H4-NO2.
Solution.
(i) Nitro group is electron withdrawing group. It deactivates the benzene ring towards the reaction with electrophile. Thus, greater the number of nitro groups attached to the benzene ring, smaller is its reactivity with an electrophile E+.
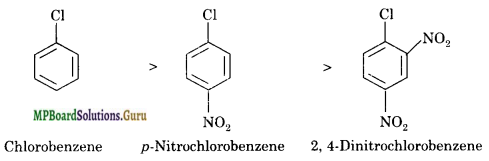
(ii) Methyl group is an electron releasing group. It increases the reactivity of benzene ring with an electrophile. On the other hand, nitro group is an electron-withdrawing group. It deactivates the benzene ring towards reaction with an electrophile. Therefore, the decreasing order of reactivity with an electrophile E+ is
Question 23.
Out of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
Solution. Out of benzene, m-dinitrobenzene and toluene, toluene would undergo nitration most easily because methyl group in toluene is electron releasing group and hence, it activates the benzene ring towards electrophilic reactions such as nitration. On the other hand, nitro groups in dinitrobenzene are electron-withdrawing in nature. They deactivate the benzene towards electrophilic substitution reactions. Thus, ease of nitration in these compounds is in the order:
Toluene > Benzene > m-Dinitrobenzene.
Question 24.
Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Solution.
Anhydrous FeCl3.
![]()
Question 25.
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
Solution.
When preparation of an alkane is attempted through Wurtz reaction, a number of side products are formed. It is very difficult to separate the mixture of these products. For example, when synthesis of propane is attempted through Wurtz reaction by reaction between ethyl bromide and methyl bromide, butane and ethane are formed along with propane.
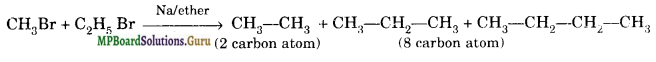
MP Board Class 11 Chemistry Hydrocarbons Important Questions and Answers
Question 1.
What are the main sources of alkane?
Answer:
Petroleum and natural gas contains mainly methane are main sources of alkane.
Question 2.
What will happen if two different alkyl halides are taken in the Wurtz reaction?
Answer:
When two different alkyl halides are used then a mixture of higher alkanes is obtained.
Question 3.
Why sometimes incomplete oxidation/combustion is considered good?
Answer:
During incomplete combustion of alkanes with insufficient amount of air or dioxygen, carbon black is formed which is used in the manufacture of ink, printer ink, black pigments and as filters.
Question 4.
Name the simplest alkane which can exhibit isomerism.
Answer:
Butane.
Question 5.
Arrange the three isomers of C5H12 in the increasing order of their boiling points.
Answer:
neo-Pentane < i.so-Pentane < n-Pentane
Question 6.
Which simplest alkene can exhibit geometric isomerism?
Answer:
But-2-ene.
![]()
Question 7.
What is the C-C-C bond angle in propyne?
Answer:
180°.
Question 8.
Arrange the three isomeric xylenes in the increasing order of their polarity.
Answer:
p-Xylene < m-Xylene < o-Xylene
Question 9.
Draw structure and IUPAC name of the starting compound used for the manufacture of teflon.
Answer:

Question 10.
How would you convert 2-butyne to Frans-2-butene?
Answer:
By reaction with sodium and liquid ammonia
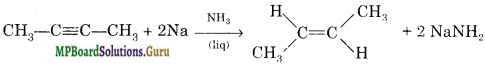
Question 11.
Give the structure of the alkene (C4H8) which adds on HBr in the presence and in the absence of peroxide to give the same product, C4H9Br.
Answer:
CH3-CH = CH-CH3
Question 12.
Arrange the following: HCl, HBr, HI and HF in order of decreasing reactivity towards alkenes.
Answer:
HI > HBr > HCl > HF
Question 13.
How will you distinguish between 1-butyne and 2-butyne?
Answer:
By reaction with ammoniacal AgNO3 solution. 1-Butyne would give white ppt.
Question 14.
How many monochlorination products are possible for
(i) neo-pentane
(ii) n-pentane
Answer:
(i) Only one
(ii) Three
Question 15.
Draw the structure of 2, 2, 4-trimethyl-hexane and find out how many each of 1°, 2°, 3° and 4° carbon atoms are there in it?
Answer:
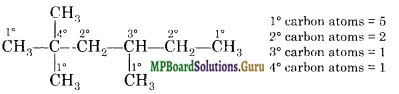
![]()
Question 16.
Which aliphatic hydrocarbon series is represented by C<sub>n</sub>H<sub>2n</sub>? Write structural
formulae of the members of this series with four carbon atoms.
Answer:
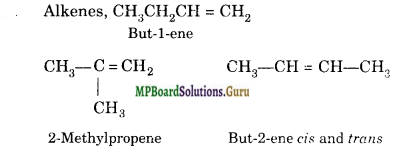
Question 17.
An alkane has a molecular mass equal to 72. Give the possible structural iso¬mers along with their IUPAC names.
Answer:
Alkanes have general formula CnH2n+2
12 x n + 1 x (2ft + 2) = 72
12n + 2fn + 2 = 72
n = 5
Thus, molecular formula of alkane is C5H12
There are three isomeric alkanes having formula C5H12 :
(i) Pentane
(ii) 2-methyl butane
(iii) 2, 2-Dimethylpropane.
Question 18.
Write IUPAC names of the following compounds.
(i) CH3CH = C(CH3)2
(ii) CH2 = CH-C -C-CH3
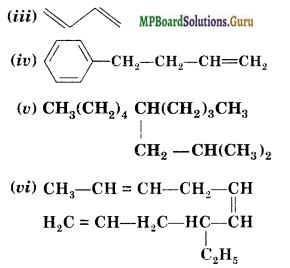
Answer:
(i) 2-Methylbut-2-ene
(ii) Pent-1-ene-3-yne
(iii) Buta-1, 3-diene
(iv) 4-Phcnylbut-l-ene
(v) 5-( 2-Methyipropyl )-decane
(vi) 4-Ethyldeca- 1, 5, 8-triene
Question 19.
Draw all the possible structural isomers with molecular formula CG1114 and give their names. Does any of them exhibit enantiomerism?
Answer:
There are five isomers; No.
![]()
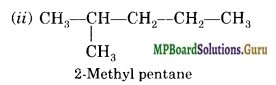
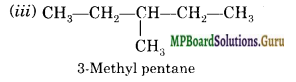
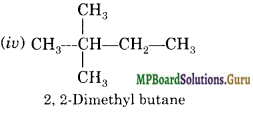
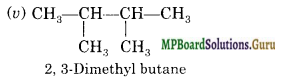
Question 20.
How many isomeric pentylenes (including stereoisomers) are possible?
Answer:
There are six isomeric pentylenes.
Question 21.
Which of the following compounds will show cis-trans isomerism?
(i) (CH3)2 C = CH-C2H5
(ii) CH2 = CBr2
(iii) C6H5CH = CH-CH3
(iv) CH3CH = CClCH3.
Answer:
(iii) and (iv). In structures (i) and (ii), two identical groups are attached to one of the doubly bonded carbon atoms.
![]()
Question 22.
Draw cis and trans isomers of the following compounds. Also write their IUPAC names:
(i) CHCl = CHCl
(ii) C2H5(CH3)C = C(CH3)C2H5.
Answer:
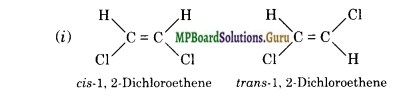
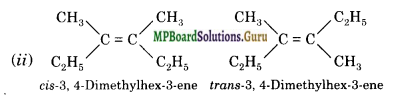
Question 23.
What are addition reactions? Which of the following will not give addition reactions and why?
(i) Ethyne
(ii) Ethane
(iii) Ethene.
Answer:
Ethane; because it does not contain multiple bond.
A reaction in which one molecule with another to form a larger moelcule with no other products.
Question 24.
What happens when?
(i) Ethyl iodide is reduced
(ii) 2-propanol is heated with cone. H2SO4 at 440 K
Answer:
(i) C2H6
(ii) CH3CH = CH2
Question 25.
An alkene is subjected to ozonolysis followed by reduction of ozonide with zinc and water. The main products formed are propanal and propanone. Predict the structure of alkene and give its IUPAC name.
Answer:
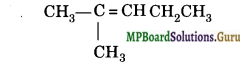
Question 26.
Draw cis and trans isomers of the following compounds:
(i) 1, 2-Dichloroethene
(ii) 1-Phenylprop-l-ene.
Answer:
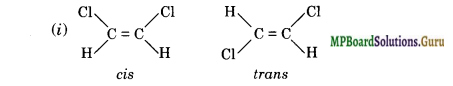
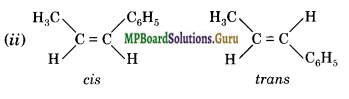
Question 27.
Which of the following compounds can exhibit cis-trans isomerism?
(i) 1, 1-Dibromoprop-l-ene
(ii) 2-Methylbut-2-ene
(iii) 1-Bromoprop-l-ene
(iv) 2-Phenylpropene.
Answer:

In (i), (ii) and (iv) two identical groups are attached to one of the doubly bonded carbon atoms.
Question 28.
Which alkane is found in coal mines and marshy places?
Answer:
Methane in gaseous form.